
The Future of Generic Nexplanon: An Interview on Women’s Health and Contraception
Table of Contents
An Interview With:
- Jason McConnell, Lab Manager, Drug Implant Development
- Onajite Okoh, Director, Drug Implant Development
- Robert W. Lee, Ph.D., SVP, Business Development
At Agno Pharmaceuticals, we’re always looking ahead to the future of drug development and the exciting innovations in this industry. For this article, we sat down with Agno’s Jason McConnell, Onajite Okoh, and Robert W. Lee to discuss the future of a Nexplanon generic and what that might mean for women’s health, contraception options, and global healthcare.
Explain Nexplanon and Its Role in Contraception

Jason McConnell: Nexplanon is a small, flexible, non-degradable implant that is inserted under the skin in the upper arm. It releases a contraceptive agent for a period of up to three years. Some ongoing studies show it may be effective for up to five years, but for now, it’s approved for three years. It works by releasing etonogestrel at a controlled rate to prevent eggs from being released. It also thickens the cervical mucus, making it harder for sperm to reach the egg. On top of that, it thins the uterine lining, making it difficult for an egg to be implanted if it were fertilized.
Because it’s a non-degradable implant, a doctor can remove it at any point during that three-year period to reverse the effects.
Onajite Okoh: Nexplanon allows women the flexibility to end contraception when they decide to. A study on Nexplanon found a clearance rate of 7.5 L/h and a half-life of 25 hours, indicating a rapid decline in etonogestrel after removal. So, the person’s hormone level should return to its baseline in a few days after the implant is removed.
Setting the Stage: Understanding Nexplanon

What sets Nexplanon apart from other long-acting reversible contraceptives (LARCs)?
Jason McConnell: It’s not only highly effective –– it has a success rate of over 99 percent ––, but it’s low maintenance once inserted. It’s in place and effective for multiple years. It’s discreet. There’s little to no chance of it becoming displaced, unlike a vaginal ring or IUD. It contains a single hormone, and it’s estrogen-free, making it suitable for women who can’t use estrogen-based contraceptives.
What demand have you seen for Nexplanon in the women’s health market?
Jason McConnell: In general, there’s always a high demand for safe, effective contraception. The demand for long-acting, low-maintenance contraception is high, too. Over the years, it’s been growing in popularity, especially in young patients or those with a busy or active lifestyle. It’s highly effective, convenient, cost-effective, and meets the needs of modern women with evolving desires.
Onajite Okoh: The global market size for subdermal contraceptive implants was valued at $1.05 billion in 2023 and is projected to grow at a CAGR of 7.5% from 2024 to 2030. A generic version of Nexplanon would introduce a more affordable alternative to the market, potentially increasing access to millions of women worldwide.
The Need and Opportunity for a Generic Contraceptive
Why is there growing interest in developing a generic version of Nexplanon?
Jason McConnell: Nexplanon has been around for a while and has a proven track record, along with a history of safety and efficacy. However, it can be expensive. You have to pay for an implant and pay for a doctor to insert it. If a patient doesn’t have insurance, those costs can be overwhelming. The patent expires in the next couple of years, which makes it the ideal time for manufacturers to begin developing a generic, less expensive alternative, not only for the US but also to pack the global market with a cheaper, more accessible alternative.
Onajite Okoh: There are a few primary market drivers for developing a generic Nexplanon: cost reduction, increased access in low- and middle-income countries, increased global support for women’s health, and expanded market penetration. A generic version of Nexplanon addresses all these factors in a way that’s cheaper and more accessible to more women in the US and globally.
From a public health perspective, how could a generic option impact access and affordability?
Jason McConnell: The lower cost would significantly increase availability and expand access. Not only does a generic address upfront costs for patients that would switch from the reference listed drug (RLD, i.e., Nexplanon), but in low-income areas, it would relieve reliance on other methods that are highly dependent on patient compliance. A wider availability at a lower cost for a long-action option would lead to lower costs for clinics and public healthcare facilities and resultant long-term savings. That resource conservation would empower those clinics and facilities to provide better care for more patients in need. An affordable, accessible generic allows patients to move away from less reliable methods of contraception, and the number of unintended pregnancies is reduced, which lessens the strain on the healthcare system as far as unintended pregnancies are concerned.
Onajite Okoh: Think about developing countries where women have fewer rights; a generic Nexplanon provides them options to control their body and their reproductive choices. Certain contraceptive forms are taboo in some cultures; for example, a vaginal ring or having your male partner use a condom is not the norm, nor is it culturally accepted in some contexts. A Nexplanon generic provides effective contraception without disrupting cultural norms or stigmatizing women seeking safe, effective birth control options.
Are there any unique challenges in replicating a drug-device combination product like Nexplanon?
Jason McConnell: Even if you go into manufacturing knowing what you’re making and have the proper concentrations and materials, there are always dials to turn on the manufacturing end, especially when dealing with hot melt extrusion or when you have multiple process factors that can affect your final product. Among other things, temperature control, cooling rate, and how you store the product after manufacturing can all influence drug release. On top of that, it’s a three-year product. Long-term testing is needed to ensure the product has a similar release pattern to the RLD.
Onajite Okoh: One of the primary challenges in developing a generic is that most RLD manufacturing processes are company secrets. The manufacturing process can change the products’ characteristics, which can affect how the product performs in the body. You need a CDMO that can understand and replicate these processes to help you achieve a suitable generic product.
The Role of CDMOs in Bringing Generic Nexplanon to Market
What role does a CDMO play in the development and manufacturing of a complex product like this?
Jason McConnell: A CDMO like Agno Pharmaceuticals will take your project from the early phases to clinical supply and then scale to commercial batches to meet consumer demand. It’s important to look for a CDMO that can also provide analytical and regulatory support. That way, you can facilitate a faster and more efficient path to market without redundancies in your processes. There’s a lot of background research required to understand what you’re trying to replicate. Having a CDMO that understands the processes and materials that influence the product is critical.
Robert W. Lee: As you seek approval from the FDA, they won’t give you nuanced guidance or feedback. You can ask the FDA specific, pointed questions, present them with the ingredients, grades, and composition, and they’ll tell you whether you can proceed or not. They’re not going to explicitly tell you what to do or not to do; it’s up to you to match the attributes of the generic product. You need a CDMO that can help you articulate your development plan and identify potential gaps or issues before you get to your FDA meetings.
Onajite Okoh: Agno is a drug substance and product CDMO that provides a one-stop shop for these products. We can make or source APIs, source polymers – we have strong relationships with our vendors, and then fully develop them for commercial manufacturing.
How early should a CDMO be involved in the planning process for a generic version?
Onajite Okoh: As early as possible. You want to leverage the processes used to develop the prototypes to ensure those processes can translate to commercial manufacturing. Those processes should be scalable and reproducible, and the CDMO should support your success at that stage in development.
Robert W. Lee: Start early. That’s how we approach all our drug development programs and projects. At Agno, we like to get in as early as possible to ensure we’re building in quality from the very beginning, so our clients don’t have to unlearn any bad practices. We have the experience and expertise to look downstream to minimize risk and give clients a clear path from design through to commercial.
How important is experience in drug-device combination products when choosing a CDMO partner?
Jason McConnell: If you’re developing a drug-device combination product, your CDMO must have experience. Anyone with a background in thermoplastics processing can transition to the pharma space and run the equipment. But understanding the nuances of how the processes and parameters affect the products and how polymers and excipients interact together and with the API to give you a targeted drug release is key. Not every CDMO can offer this; it’s a skill that must be developed over time and experience.
How can Agno help accelerate innovation in this space while keeping costs down for patients?
Onajite Okoh: Agno has established facilities, engineering controls, and expertise in-house to handle and incorporate sex hormones, which not every CDMO offers or has the capability to. Equipment for this industry and these projects can have very long lead times—isolators can have a 15 – 24-month lead time. We already have the equipment in place to manufacture generic Nexplanon prototypes that can be used in GLP studies. Through the development phase, we can quickly and cost-effectively get the client into animal and GLP studies with existing facilities and expertise. Because we have this equipment in-house, we can reduce our clients’ capital expenses even before they show a promising formulation.
The Future of Generic Nexplanon
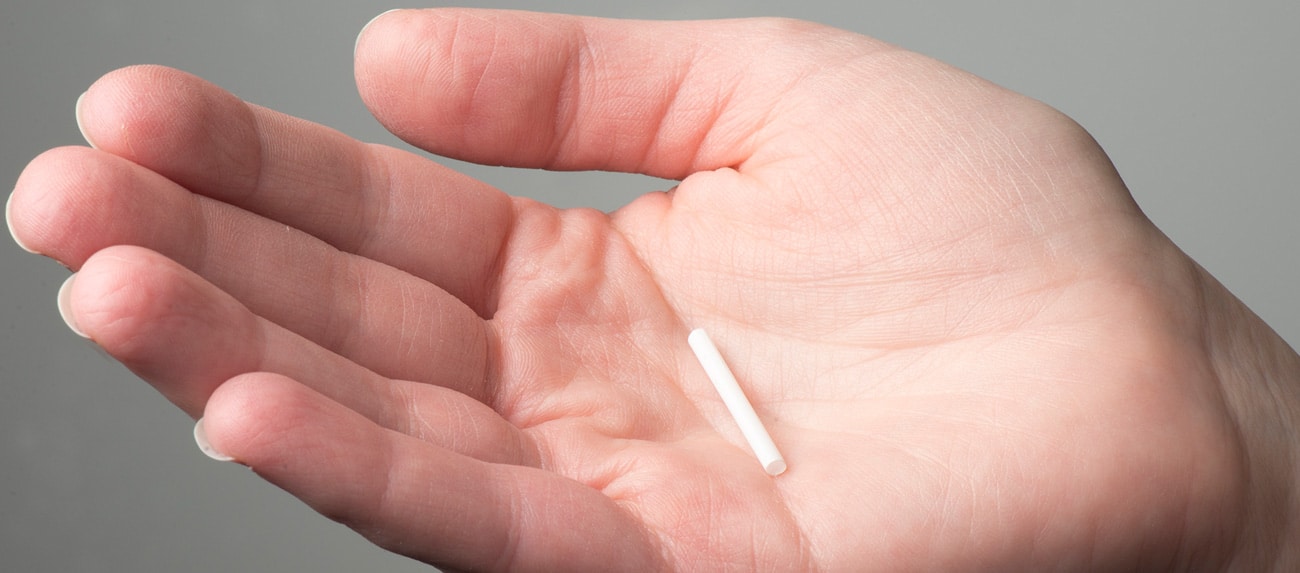
What is the anticipated timeline for a generic Nexplanon to reach the market?
Onajite Okoh: The US patent for Nexplanon expires in 2027, and the patent for the applicator expires in 2030. Already, some companies are looking to develop a generic. We’re about three to five years out from developing a generic and seeing it come to market.
What should stakeholders—manufacturers, providers, and patients—be watching for in the coming years?
Onajite Okoh: Market volatility is a constant factor, as are new drugs capturing some of the available market share.
Robert W. Lee: We can expect more seamless collaboration between CDMOs and suppliers. Also, keep in mind the potential for competition. You want to be the first generic out there, but there’s a high probability that a competitor could launch a generic around the same time. Timing is everything in generic drug development; now is the time to develop so you’re ready before the patent expires. A good CDMO matters for this. Agno reduces risk and ensures you’re supported by seasoned chemists and experts, state-of-the-art infrastructure, and a proven track record that helps you execute a successful drug development project.
How can companies prepare now for the shift to generics in the contraceptive implant space?
Onajite Okoh: Agno Pharmaceuticals has developed and identified what we consider an acceptable generic version of Nexplanon. Compared to the RLD, our prototype meets all chemical and structural criteria. We have the infrastructure and expertise to manufacture and supply these products.
Robert W. Lee: The DID group has a breadth of knowledge and experience and the ability to handle projects across the development spectrum from inception to commercialization. We act more like members of the client’s research team, not contractors. We take more ownership and responsibility for a successful outcome.
Onajite Okoh: We consider ourselves partners in the success of our clients’ products. As of today, there are no approved generics for Nexplanon, so it’s a race to the finish line. What we offer in this race is that we’ve already developed a walking prototype; we just need a partner to take it the rest of the way.
Agno Can Help In Every Phase Of Your Project
The team at Agno Pharmaceuticals prioritizes client success. Your win is our win, and that mentality shines through at every phase of your drug development project. No matter what you’re exploring or developing, our answer is always “Yes,” ensuring superior quality and compliance from design to commercialization.
Reach out to the team at Agno Pharma with any questions or to talk about your project.