Core Competencies
Learn more about what Agno Pharmaceuticals can do to help you accomplish your goals.
Technology Feasibility Screening
Biopharmaceuticals Formulation Development and Optimization
Solubility & Bioavailability Enhancement
Microspheres, Suspensions, Emulsions, Lyophilization, Liquid Fill
Micro and Nanotechnology
Oral Formulations

Using rigorous technology feasibility screenings, we identify optimal approaches for each formulation project
Our expertise includes enhancing solubility and bioavailability of drug compounds through innovative techniques. We specialize in micro and nanotechnology applications, enabling precise control over particle size and distribution. Agno Pharma’s proficiency extends to biopharmaceutical formulation development and optimization, encompassing microspheres, suspensions, emulsions, lyophilization, liquid fill formulations, spray drying, and oral dosage forms. With a focus on precision and innovation, our facility is dedicated to delivering tailored solutions that meet the highest standards of quality and efficacy in complex pharmaceutical formulations.
Additional Services
Our dedicated feasibility programs are meticulously designed to expedite product development in these pivotal areas, leveraging state-of-the-art technologies to provide early-stage support in efficient and cost-effective packages.
- Nanomilling Feasibility Program: Our nanomilling capabilities are tailored to enhance the dissolution rates and bioavailability of poorly soluble BCS Class II and IV compounds. Through scalable solutions, we enable efficient development paths that optimize drug performance and patient outcomes.
- Microsphere Feasibility Program: Designed for controlled release applications, our microsphere feasibility program excels in encapsulating large quantities of APIs. This technology is suitable for parenteral and ophthalmic administrations, ensuring precise and sustained delivery of therapeutic agents.
- Long-Acting Implant (LAI) Feasibility Program: Responding to the pharmaceutical industry’s demand for sustained-release platforms, our LAI feasibility program focuses on developing long-acting implants. Products like Ozurdex® exemplify the success of polymeric LAIs in treating conditions such as macular edema, highlighting their potential for prolonged therapeutic efficacy.

Agno Pharma is committed to navigating complex formulation challenges and guiding products from initial concept through to commercialization. Our tailored feasibility offerings are designed to meet specific needs across various therapeutic areas, ensuring robust development pathways and successful market introductions. Whether you’re aiming to improve drug performance, enhance patient compliance, or explore novel delivery systems, Agno Pharma provides the expertise and capabilities to achieve your pharmaceutical development goals effectively.

We specialize in overcoming the challenges of poor water solubility and low bioavailability, which afflict over half of marketed APIs and up to 90% of new drug candidates. We understand that formulating BCS Class II and IV compounds requires tailored approaches rather than one-size-fits-all strategy. That’s why offering a comprehensive range of techniques to enhance solubility and bioavailability helps ensure that your drug products achieve their therapeutic potential.
Our expertise spans physical modification services such as nanomilling, micronization, and amorphous solutions. Nanomilling involves reducing API particle size via a high energy milling process in a liquid medium, significantly improving dissolution rates. Micronization, achieved through jet milling, creates micron-scale particles for enhanced dissolution. Amorphous solutions stabilize APIs in a non-crystalline state through techniques like hot melt extrusion and spray drying in the presence of suitable polymers.
Our team of experts excels in encapsulation techniques such as micelles, polymeric nanoparticles, solid-lipid nanoparticles, and inclusion complexes. These methods encapsulate APIs within structures that enhance stability and solubility, facilitating effective drug delivery systems tailored to your specific needs.
Whether you are tackling formulation challenges for new drug candidates or seeking to improve existing products, Agno Pharma is your partner of choice. Our commitment to innovation and quality ensures that we deliver customized solutions that optimize drug performance and pave the way for successful commercialization. Explore our comprehensive range of services and start your project with us today to transform your formulation goals into reality.
Agno Pharma stands at the forefront of micro and nanotechnology in the pharmaceutical field. Rather than adopting a one-size-fits-all approach, we customize programs based on the physicochemical properties of each molecule and the overarching objectives of the drug product.
Our scientists have extensive experience in developing microsphere and nanoparticle suspensions, and we are uniquely positioned to tech transfer proprietary or academic technologies/processes and bring them into GMP production. When it comes to solubility enhancement, we are the leaders in the use of nanomilling, employing both commercial milling equipment and our own proprietary aseptic mills (SteriMill™).

This flexibility in formulation and process design combined with access to cutting edge technologies ensures the best possible outcome for your drug product.
We harness decades of expertise in advanced micro and nanotechnology to tackle complex formulation challenges and drive innovative drug development solutions. Our comprehensive capabilities include high-energy media milling, micronization, emulsion-solvent evaporation, homogenization, microfluidization, tangential flow filtration, spray drying, and lyophilization. These technologies enable us to enhance bioavailability, improve stability, formulate higher doses, enhance safety and patient compliance, control release rates, and protect unstable APIs from degradation. We continue to lead nanoparticulate projects, including those involving highly potent APIs and controlled substances. Agno Pharma is uniquely equipped with aseptic and sterile capabilities for nanomilling, allowing us to perform commercial production under stringent cleanroom conditions.
Agno Pharma understands the physical and chemical properties that make biopharmaceuticals different from small molecules. During formulation, our biopharmaceutical company considers the molecule’s stability requirements, its tertiary structure, and the need to retain biological activity.
The clinical and commercial success of biopharmaceutical products depends on the development and validation of robust processes. We are experienced at incorporating these fragile molecules into dosage forms for virtually any route of administration while maintaining biological activity. As with all our formulation offerings, we provide a personalized approach to find the best solution for our clients.
We excel in developing and manufacturing complex large molecule therapies such as proteins, peptides, and oligonucleotides. Our capabilities cover the entire lifecycle—from formulation and characterization to scale-up and commercial manufacturing. Given the challenges of terminal sterilization for biopharmaceuticals, our ISO 5 suites feature advanced isolator technologies, supporting aseptic processing for biologics with lyophilizers in both clinical and commercial settings.
We specialize in customizing delivery profiles to enhance bioavailability and develop long-acting dosage forms, crucial for patient compliance and product lifecycle management. Our expertise extends to 505(b)(2) products and intellectual property strategies. Agno Pharma is also a leader in meeting the demand for lyophilized complex drug products, including biologics and long acting injectables, with lyophilizers equipped for aseptic processing.

We support a wide range of dosage forms, focusing particularly on parenteral formulations essential for biologic drug delivery. Our analytical capabilities include advanced biologic characterization techniques such as protein assays and ELISAs, conducted by a skilled team using specialized equipment. This expertise ensures rigorous development, validation, and quality control of biopharmaceutical methods tailored for large molecules, including stability testing and in vitro release assessments.
We provide for the development of a variety of dosage forms. We’ve developed and manufactured these formulations over the decades, including PLGA microspheres and also nanoemulsions for US Phase 2 clinical trials. We offer services from feasibility screening through manufacture of GLP test articles, GMP clinical trial materials, and potentially commercial production. This capability to span the life cycle of product development offers our clients reduced risk plus a cost-effective path to their ultimate objective.
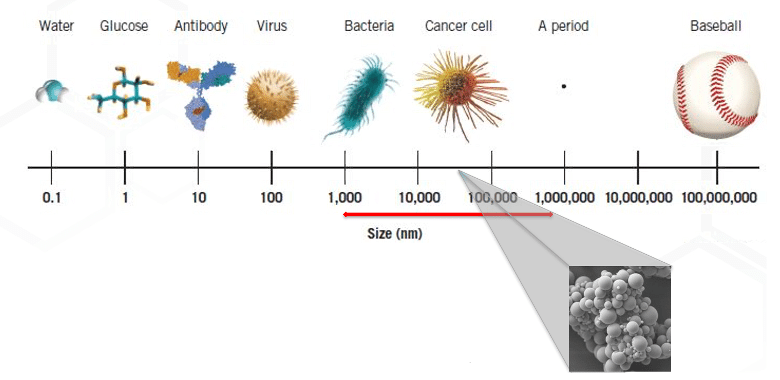
Most of the small molecules that clients bring to us have low water solubility. We have technologies well suited to optimize the performance of dosage forms. These include:
- Nanoparticle suspensions via high energy milling
- Amorphous API via
- Hot melt extrusion
- Spray drying (we have small-scale systems for screening experiments and are adding larger-scale GMP manufacturing in the near future).
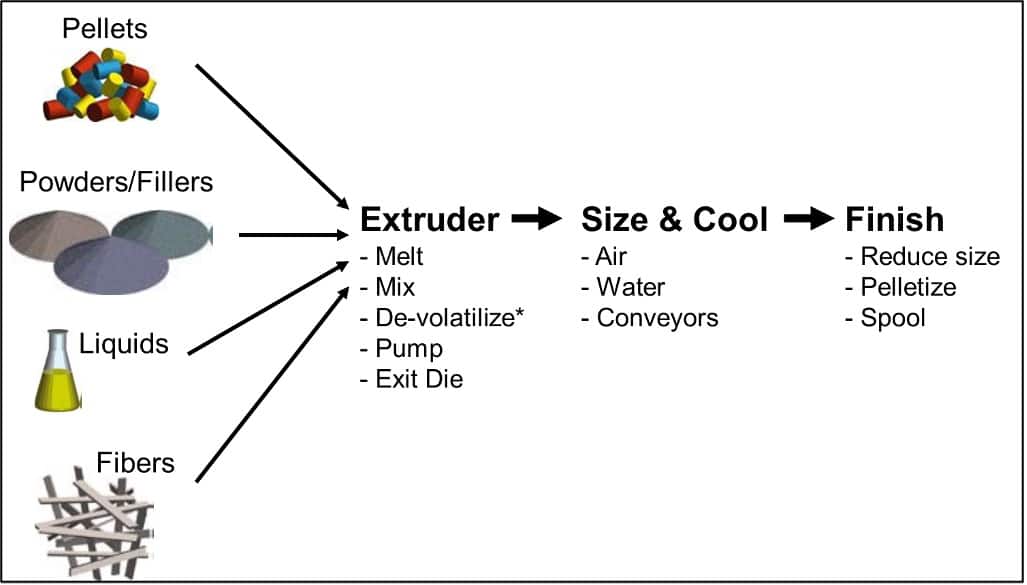
Our facility can support preclinical development through GMP manufacture for early clinical trials. Additionally, our well-equipped analytical labs can fully support characterization of these dosage forms plus GMP release testing and ICH-compliant stability studies.