
Skin and Nail: Barrier Function, Structure, and Anatomy Considerations for Drug Delivery
Table of Contents
Drug delivery for both skin and nails is an area receiving ever-increasing attention. To effectively deliver active pharmaceutical ingredients (APIs) across the skin (transdermal delivery) or nail (transungual delivery) it is necessary to consider the anatomy and physiology of these barriers. With this information in hand, one can more effectively utilize drug delivery approaches to maximize the effectiveness of the API – getting the right amount to the right place at the right time. The advantages of local delivery include, among others, minimized systemic toxicity, high local drug concentrations, avoidance of first pass issues and cost.
Topical delivery of systemic therapeutics also offers benefits but present a greater technical challenge. Among the benefits are first pass avoidance, convenience and sustained release.
Skin
The skin is the largest organ in the body, making up 12 – 15% of body weight and with a surface area of 1 – 2m2. Not surprisingly, the skin has a complex architecture that can vary depending on body location (Figure 1).
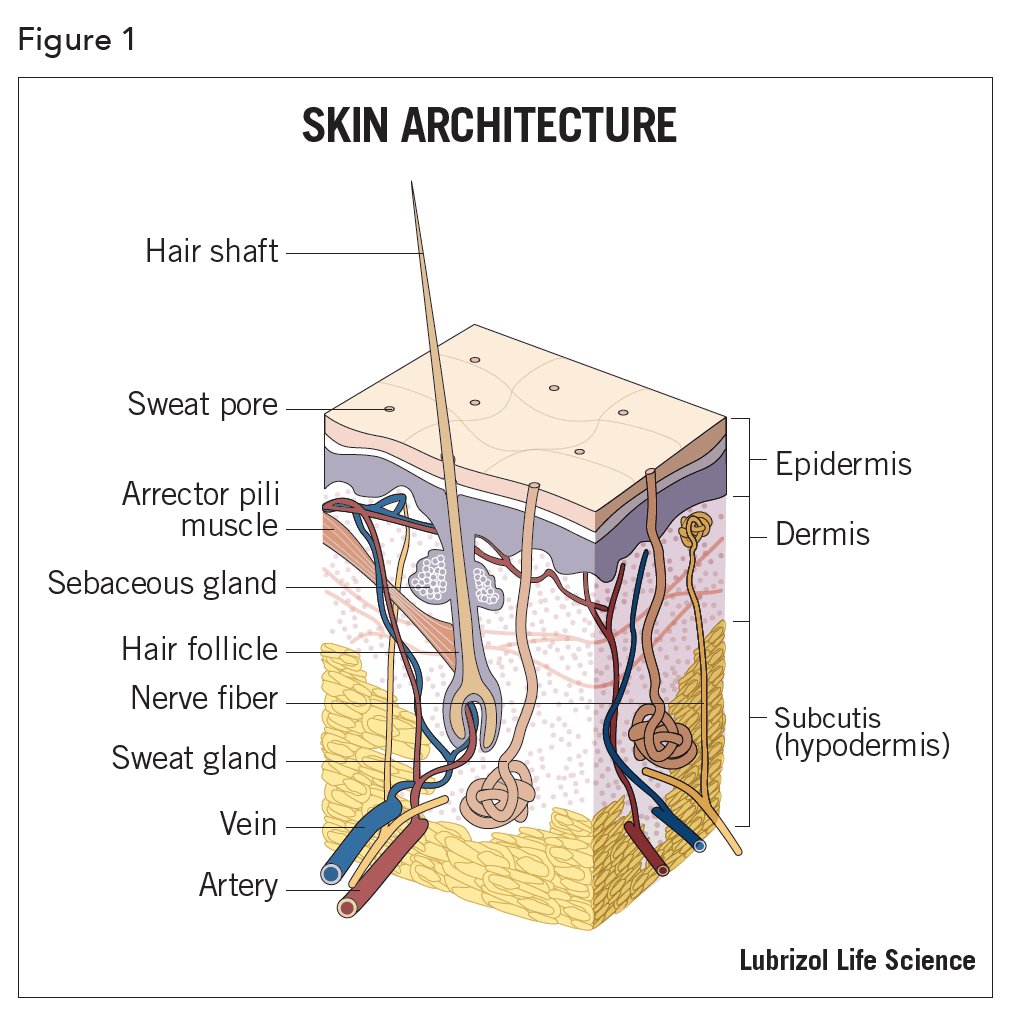
There are 3 distinct regions in the skin. From outermost inward, they are the epidermis, dermis and subcutis (hypodermis). The epidermis itself has five regions and can range in total thickness from 0.5 mm (eyelid) to 1.5 mm (palms and soles) (Figure 2).
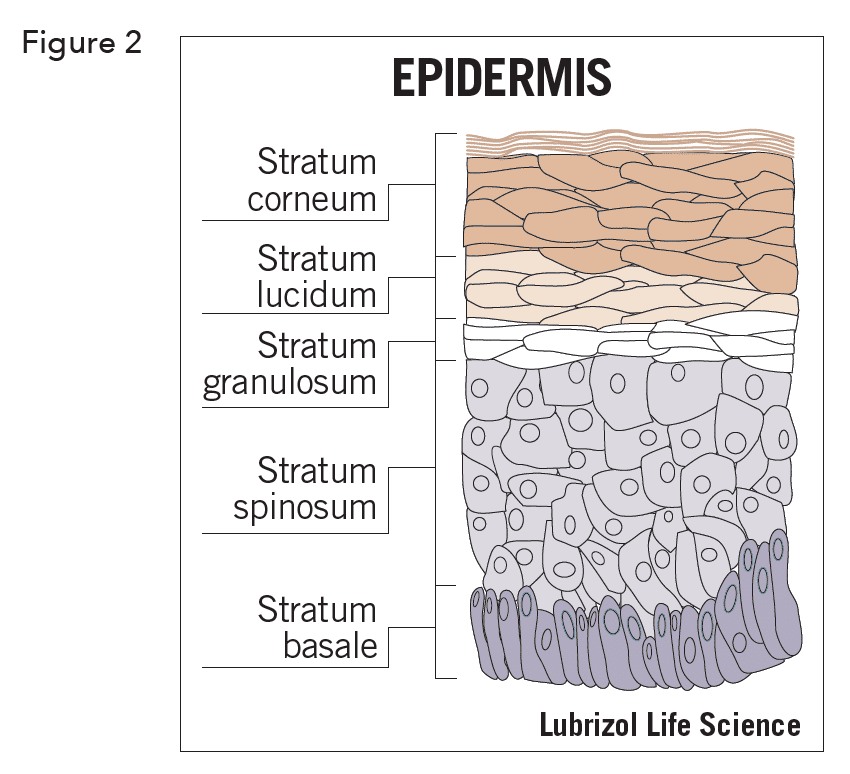
The most important function of the skin is to provide a selectively permeable barrier. The outermost region of this barrier is called the stratum corneum (SC), a dynamic structure with cells migrating in a deep to superficial direction as they mature. The SC, also called the horny layer, consists of cells called corneocytes that are embedded in a multilamellar, lipid- enriched extracellular matrix. The SC comprises, depending on location, approximately 15 layers of horny cells. The transit time of the horny cells across the epidermis is approximately 1 – 2 weeks. It takes approximately 24 hours to form one layer and the SC is completely renewed about every 15 days. In addition to the skin, there is a relatively large area of accessible mucosal tissue. This includes the conjunctiva, nasal and oral cavities, respiratory tract, digestive tract, vagina, urethra and rectum. Each of these areas has a unique histology that, for the most part, make them more permeable to many drugs compared to skin or nails. For instance, these surfaces may have a substantially reduced keratinized layer, or lack one altogether, which greatly reduces barrier function. Being at the interface of the body and internal spaces, mucosal areas have highly developed transport mechanisms for dealing with foreign bodies, such as the ciliated epithelium found in the respiratory tract. Many mucosal surfaces have a single-cell thick epidermis and most are highly vascularized. Of course, mucosal surfaces are “wet” which has obvious implications for drug transport. Mucosal surfaces have unique immunologic properties, specific to the anatomical site. Several mucosal surfaces are already regularly exploited for local and systemic drug delivery.
There are three routes for transit of substances through the epidermis: Appendageal, Transcellular, and Intercellular. Appendageal transport refers to the use of hair follicles and sweat glands. As areas of discontinuity in the SC, they can serve as a pathway for API penetration. The significance of their contribution to percutaneous absorption has been extensively investigated. Although it is estimated that these appendages account for no more than 0.1% of the total skin surface, they cannot be neglected in considering possible routes of percutaneous absorption. For instance, iontophoresis, which is a non-invasive method for transdermal delivery of charged APIs using electromotive force, appears to dramatically increase the flux of compounds via hair follicles.
The transcellular pathway involves the movement of the API across the epithelial cell. The outer membrane of an epithelial cell may be regarded as a layer of lipid, surrounded on both sides by water. Low molecular weight and lipophilic APIs can gain systemic access transcellularly by passive diffusion across the epithelial cells. In the process of passive diffusion, lipid-soluble substances move into the lipid membrane according to their lipid/water partition coefficient. The structural features of compounds that determine permeability are related to hydrophobicity and molecular volume. In transcellular passive diffusion, the epithelium is assumed to act as a simple lipophilic barrier through which APIs diffuse and the rate of diffusion correlates with the lipid solubility of the API.
Lastly, drugs can travel between cells via intercellular channels. It is generally accepted that the extracellular SC lipids play a key role in limiting the diffusion of compounds through the SC. Lipids exist in bilamellar structures in the SC extracellular space, each layer separated from the other by a thin water sheet associated with the polar head group of the lipids. The intercellular lipids are organized into lamellae, which run parallel to the skin surface. Intercellular lipid is required for a competent skin barrier and forms the only continuous domain in the SC – a structural feature that may account, in part, for the barrier properties of the skin. The molar ratio of the barrier lipids was found to be cholesterol ester (15%), saturated long chain free fatty acids (16%), cholesterol (32%) and ceramides (37%). Chemical penetration enhancers generally act through disruption of the extracellular structure.
The three pathways described above are not mutually exclusive and permeation will be determined by the area/volume of the pathway, partitioning, and diffusivity of the API in each pathway. Transport across the SC, for most compounds, involves intercellular lipids. However, it does not exclude the possibility that APIs also penetrate the corneocytes. The molecular basis for the penetration pathway across the SC should be considered in terms of its morphological structure and material properties. Both the composition and structure of the SC influences its material properties, which in turn determines the pathway for diffusion as well as the solubility and diffusivity of API within the barrier.
It is essential to understand the inherent ability of an API to penetrate the SC before studying to what extent the penetration can be enhanced or reduced by appropriate choice of formulation. The relationship between the structure of an API and its permeability across the skin provides insight into the nature of the permeability barrier and is directly applicable to the prediction of percutaneous absorption.
Nails
Nails and claws of mammals are specialized epidermal derivatives which protect the delicate tip of fingers and toes against trauma and act as tools or weapons. Human fingernail gross anatomy consists of three structures. Starting from the outer structure, they are the nail plate, the nail bed, and the nail matrix (Figure 3)1.
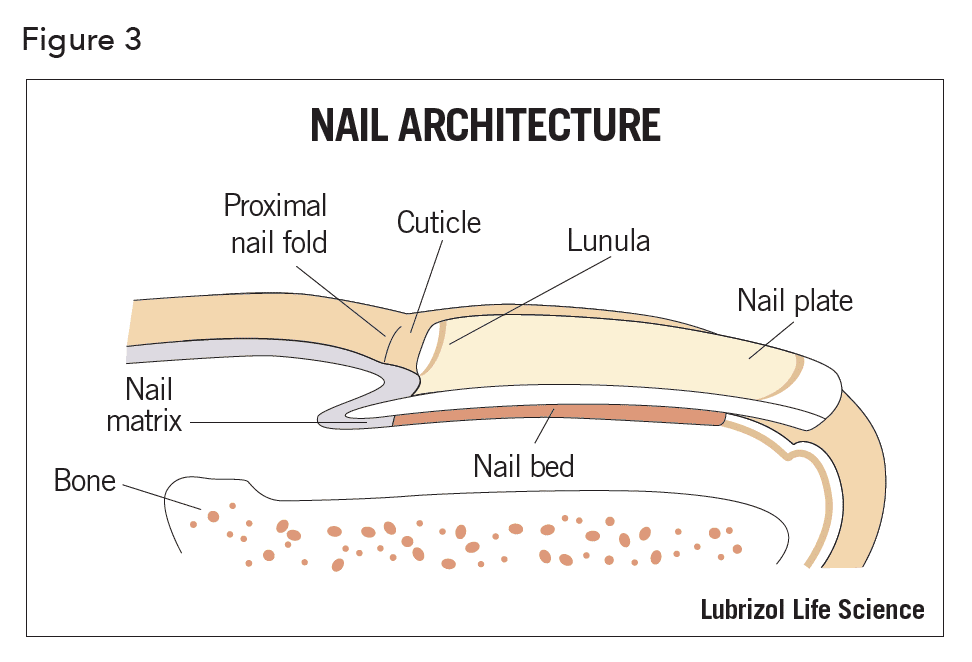
The nail plate is a thin (0.25 -0.6 mm for fingernails and up to 1.3 mm for toenails), hard, yet slightly elastic, translucent, convex structure and is made up of approximately 25 layers of dead keratinized, flattened cells. They are tightly bound to one another via numerous intercellular links, membrane-coating granules and desmosomes, which are cell structures specialized for cell-to-cell adhesion and randomly arranged on the lateral sides of plasma membranes. The fingernail has a three-layer structure (outer to inner) – the dorsal, intermediate, and ventral layers, with a thickness ratio of approximately 3:5:2, respectively. The dorsal outer layer is dense and hard, consisting of cornified keratin only a few cells thick (approximately 200µ). The cells at the dorsal surface of the plate overlap and produce a smooth surface. The intermediate layer, in contrast to the dorsal layer, shows highly fibrous structure oriented perpendicular to the direction of nail growth and constitutes roughly 75% of the plate’s thickness. The keratin fibers are thought to be held together by globular, cysteine-rich proteins, whose disulfide bonds act as glue. The intermediate layer is believed to be both the softest and thickest. The ventral layer is very thin and consists of a few layers of cells which connect the nail plate to the nail bed below.

The growth rate of nails is highly variable among individuals, with average values of 3 mm per month for fingernails and 1 mm per month for toenails. A normal fingernail grows out completely in about 6 months, whereas it takes a toenail about 10 – 12 months1.
The physicochemical properties of the human nail plate exhibit a marked difference to that of epidermis, resulting in very different permeability characteristics. Whereas the SC behaves as a lipid barrier to the permeation of low molecular weight chemicals, the nail plate exhibits behaviors similar to that of a hydrogel with high ionic strength and, indeed, the structure of human nail has been likened to a hydrophilic gel membrane2. The lipid content of the nail is reported to be low, at between 0.1 – 1%, and the nail is much more susceptible to water loss than the lipid-rich skin. Despite the reported hydrophilic properties of the nail, hydrophobic compounds also have been shown to diffuse into and through this barrier. For example, Walters and coworkers reported that long chain alcohols also permeate through the nail via a lipidic pathway3.
For effective transungual drug therapy, permeation must be enhanced. This can be achieved by disrupting the nail plate using physical techniques or chemical agents. Alternatively, drug permeation into the intact nail plate may be encouraged, for example, by iontophoresis or by formulating the drug within a vehicle which enables high drug partition into the nail plate.
There are several physical techniques that have been shown to enhance transungual delivery4. These include nail abrasion (manual and electrical), acid etching, ablation by lasers, microporation, application of low-frequency ultrasound and electric currents, and chemicals (thiols, sulphides, hydrogen peroxide, urea, water, enzymes).
References
- V. Gupchup, J.L. Zatz;J. Cosmet. Sci., 50, 363-385 (1999).
- Murdan: InternationalJournal of Pharmaceutics, 236, 1-26 (2002).
- A. Walters, G.L. Flynn, J.R. Marvel: J. Pharm. Pharmacol., 37, 771-775 (1985).
- R. Prausnitz, R. Langer;Nature Biotechnology, 26:11, 1261-1268 (2008).