
Surfactants
Table of Contents
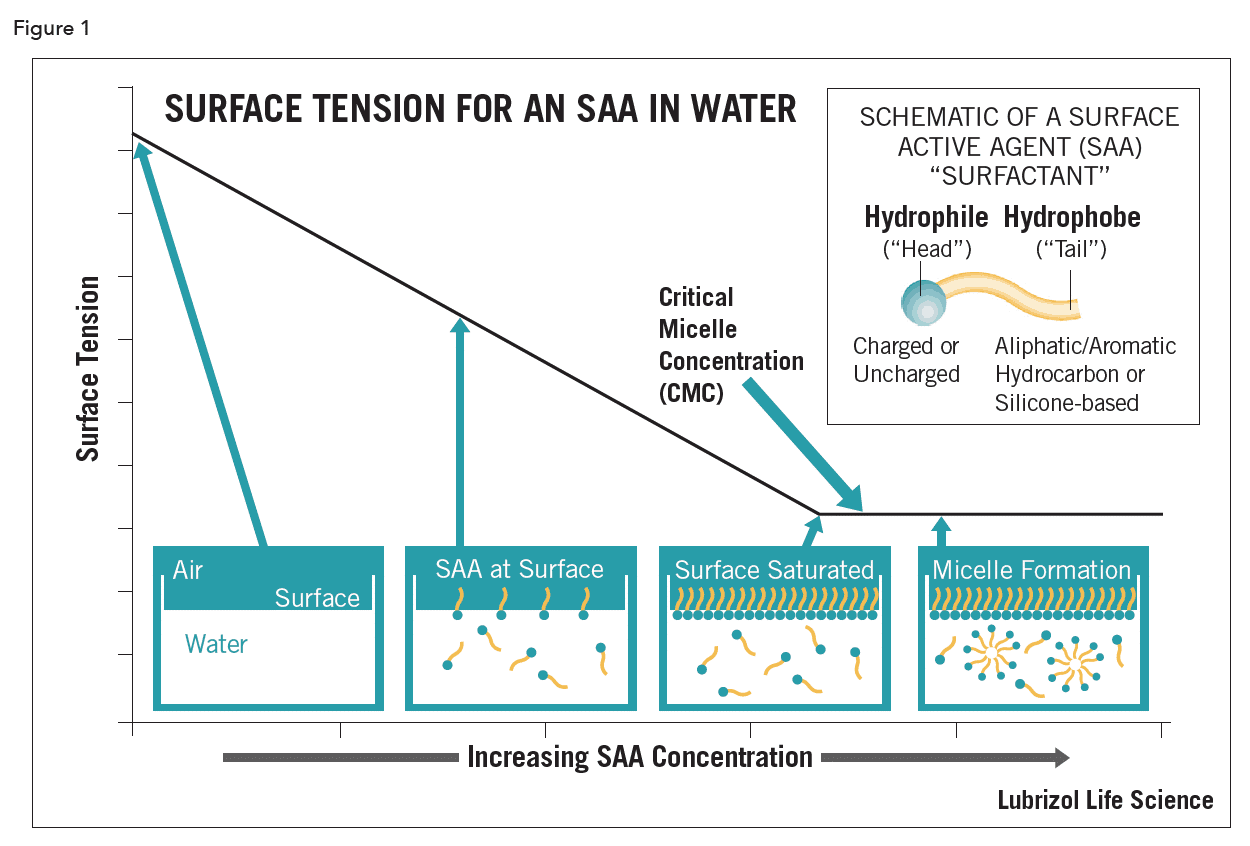
The word surfactant is a contraction of the term surface active agent (SAA). To be surface active a molecule needs to have two mutually insoluble portions. Surfactants, also known as tensides, are amphiphilic as they have a hydrophilic polar head group combined with a hydrophobic tail (Figure 1)1,2. SAAs are broadly classified according to their polar head group nature: nonionic (neutral), cationic (positively charged), anionic (negatively charged), or amphoteric (negatively and positively charged). An SAA is classified as being amphoteric only if its charge changes as a function of H+/OH- concentration, i.e., a positive charge in acidic media, a negative charge in basic media, and a zwitterionic (or isoelectric) species at intermediate pH values. Living systems, including cells, make use of naturally occurring amphoteric surfactants such as lecithin and phospholipids. A separate category now exists for SAAs where the hydrophobe is not hydrocarbon, but silicone-based (i.e., dimethicone copolyol)3.
Thousands of SAAs are commercially available, with many in each class. The structure of the SAA determines its functionality. The range of SAA functions is very broad – about thirteen in all. Some of the more common are: detergents, emulsifiers, wetting agents, dispersants, and solubilizers, with the first two being the most important applications. From a physicochemical perspective, each function is discrete and a particular SAA class can serve multiple functions but usually has only one or two at which it excels.
To choose which SAA is best requires a thorough understanding of the particular application. One example is the process of laundering and dishwashing, which ideally necessitates several discrete functions from the same molecule including wetting, emulsification, dispersion, and solubilization. However, the best wetting agents are not the best overall detergents and vice versa. Wetting agents lower the surface tension (ST) of a fluid and also lower the interfacial tension (IT) with another fluid or solid. Detergents are cleaning agents that remove dirt and grease from porous and non-porous surfaces. For a homologous series of SAAs such as the alkyl sulfonates, optimum detergent action is achieved with the C14 tensides and optimal wetting by the C8 tensides. Cationic SAAs do not function well as detergents, but are exceptionally substantive hence their use as fabric, hair, and skin conditioning agents. Most non-ionic SAA are much less effective in dirt removal than anionics; one exception is poly(ethylene oxide)-based nonionics. Detergent action is dependent only on the concentration of monomeric SAA molecules but solubilization of dirt requires associated tenside structures called micelles. There is no correlation between the detergent action and dispersing power of an SAA; some tensides that are excellent dispersants are bad detergents. It is for all these reasons that commercial laundry detergents are formulated to contain mixtures of different SAAs. The same reasoning needs to be applied to any application where SAA use is considered.
Amphiphilic molecules can increase transport of active pharmaceutical ingredients (APls) across the skin (a hydrophobe of C18 chain length is most favorable) by modulating the organization of stratum corneum (SC) lipids and by the formation of separate domains within the intercellular lipid regions. However, SAAs that function as detergents must be avoided since they are powerfully membrane disruptive.. Another important property to remember with regards to biologic systems as that SAAs increase the osmotic pressure of liquids.
Properties and Characteristics
SAA structure endows them with unusual physicochemical properties such that they tend to adsorb at any interface with the hydrophilic group located in one phase and the hydrophobic group in the other. The first important distinguishing property of an SAA is its ability to lower the ST at a liquid/air interface or the IT between two immiscible liquids (Figure 1).
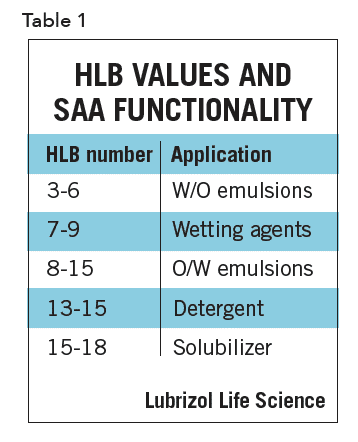
It is this latter property that allows an SAA to function as an emulsifier. The amphiphilic nature of many SAA emulsifiers (particularly nonionics) can be expressed in terms of an empirical scale of the HLB (hydrophilelipophile balance) numbers, which range from 1 to 205. SAA functionality tends to fall into rough ranges of HLB values (Table 1).
An SAA does not have to be completely soluble and may lower the ST or IT by spreading over the interface. They also lower the contact angle of a liquid in contact with a solid substrate, thus allowing it to act as a wetting agent.
Micellization and Liquid Crystal Structures
A second important property of SAAs is that, above some specific narrow concentration range (depending on molecular weight and shape of the hydrophobe), they will spontaneously self-associate through an aggregation process known as micellization6. The concentration at which these micelles form is termed the critical micelle concentration (CMC) (Figure 1). Micelles contain from tens to hundreds of molecules referred to as the aggregation number. In water, the hydrophobic chains are oriented inward, away from the aqueous phase. This is relevant for drug delivery of poorly water-soluble APIs as very hydrophobic APls can be readily solubilized within micellar systems. “Inverse” micelles are formed when the liquid is nonaqueous; in this case, water-soluble APls can be solubilized in oils. Such micelles can also sequester very high electrolyte levels (2M NaCl).
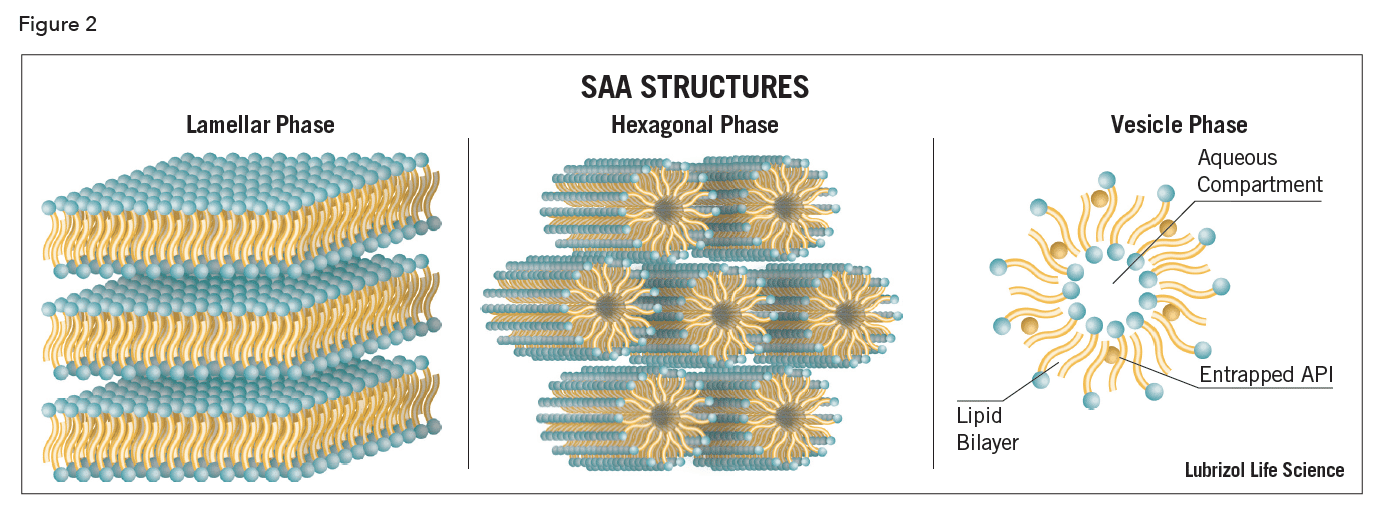
At higher concentrations, surfactants may also associate to form mesomorphic phases, i.e., a state intermediate between the liquid and crystalline, termed lyotropic liquid crystals (LCs). Lyotropic structures range from cubic to a hexagonal cylindrical array to lamellae (Figure 2). The rheological (viscoelastic) properties of these LC structures vary considerably, from shear thinning to thixotropic, and have been used with great success in food emulsions. A notable feature of many LC phases is their ability to incorporate large amounts of very hydrophilic to very hydrophobic molecules and to form very small or very large particles. The protective properties of these LC systems are useful for oral administration where an API must pass through the stomach and is sensitive to hydrolysis or degradation.
Vesicles
Vesicles are hollow colloidal-size spheres constructed from amphiphilic SAAs. A wide variety of natural lipids, in particular, phosphatidylcholine (PC) or synthetic non-ionic surfactants can be used in their preparation. These are referred to as liposomes and niosomes, respectively. These amphiphilic SANs can form – in the presence of excess water – one (known as unilamellar vesicles) or more (multilamellar vesicles) concentric bilayers that surround an equal number of aqueous compartments. Both water-soluble and hydrophilic compounds can be trapped in the internal aqueous compartments while water-insoluble and lipophilic compounds can be trapped within the vesicle bilayer or between the bilayer and the aqueous phase (Figure 2). Liposomal systems have attracted attention because they can provide skin transport enhancement and pH-responsive controlled-release characteristics7. The lipid composition and thermodynamic state of the bilayers play a crucial role in the effect of vesicle interaction with the SC and also on drug transport rate across skin.
Surface Activity of APls
APIs themselves are frequently amphiphilic and can be surface active. One potential deleterious side effect is foaming, especially in high shear mixing. Both an APl’s pharmacokinetics and pharmacodynamics are somewhat affected by its surface activity. Note that the presence of a surface-active API in a formulation could affect the type of structure formed by a primary surfactant, the excipient polymer system, and the structure’s stability.
Surfactants as Dispersing Aids
The act of dispersing an API into a liquid entails overcoming the various binding forces between particles by use of both physicochemical and mechanical means. The first step is to completely wet the powder using a suitable SAA4. Only a very low concentration of a wetting agent is needed (ca 0.01%). In the second stage – that of comminution and distribution of particles in the liquid – a distinction must be made between massive (crystalline) particles and agglomerated/aggregated materials. The former materials are usually harder and need high shear pulverizing to break them down into smaller particles. The latter process is one of separation of the agglomerated material using deflocculants. Here charged tensides are useful because they can promote internal wetting; the charge must be the same sign as the charge on the particles being dispersed. SAAs that are good deflocculants must be capable of significantly reducing the contact angle between solid and liquid but only slightly lower the liquid surface tension (to ca 60 mNm-1). This distinction is critical. Typical tensides are isopropylnaphthalenesulphonate (anionic) and cetyltrimethylammonium bromide (cationic).
All dispersants adsorb at the solidliquid interface and can prevent primary particles or fragments formed after comminution from re-agglomerating, but higher molecular weight polyelectrolytes are far better at it than tensides. Importantly, the two species are not interchangeable; each must be chosen with care because, depending on the fineness of the dispersion, the concentration of dispersing aid needed is 10% – 20% of the particles by mass, whereas the concentration of wetting agent needed to initially wet the unmilled solid is on the order of 0.01% or less.
Conclusion
The choice of a surfactant system is based upon the desired performance of the end-product. SAAs are most often used in combination to achieve the optimal combination of API state, stability, and appropriate delivery characteristics. Although there are frequently-used SAA systems, the best results are obtained by tailoring each system through careful consideration of the physicochemical properties of the API and the delivery goal.
References:
- J. Rosen, Surfactants and lnterfacial Phenomena, Wiley, New York (1978)
- Meyers, Surfactant Science and Technology, VCH Publishers, New York (1988)
- M. Hill (ed.), Silicone Surfactants, Marcel Dekker, New York (1999)
- McCutcheon’s Emulsifiers and Detergents and McCutcheon’s Functional Materials, MC Publishers, Glen Rock, NJ, published
- Becher, Encyclopedia of Emulsion Technology, Volume 2, Ap plications, Marcel Dekker, New York (1985)
- Tadros, Surfactants, KirkOthmer Encyclopedia of Chemical Technology Series, Wiley, London (2006)
- P. Martin and A. W. Lloyd, Basic Principles of Liposomes for Drug Use in Liposomal Dermatics, Springer-Verlag, Berlin (1992)