Glossary of Polymer Terms
Table of Contents
Polymers are used widely in the pharmaceutical industry as excipients such as binders, stabilizers, rheology modifiers, adhesives, film-formers, etc. and packaging materials. The polymer may even be the active pharmaceutical ingredient (API) such as DNA, RNA, insulin, etc. This glossary is designed to be a reference guide for better understanding of the terms used in the polymer field, specifically related to pharmaceuticals.
Architecture
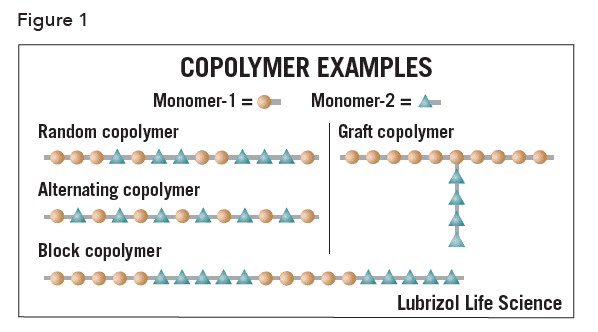
Alternating copolymer – a polymer comprising only two types of repeat unit chemically linked in an alternating sequence.
Block copolymer – a polymer comprising more than one type of monomer repeat unit, in which each type of monomer is found in a homopolymerized “block” within the polymer chain, e.g., poly(ethyleneoxide-b-propyleneoxide) (PEO-PPO, Pluronic™).
Branched polymer – a polymer molecule comprising a linear polymer backbone with one or more substituent side chains (branches) of similar chemistry, e.g., low density polyethylene.
Copolymer – a polymer comprising more than one type of repeat unit, e.g., poly(ethylene-co-vinyl acetate) (EVA).
Cyclic polymer (ring polymer) – a polymer that is a continuous chain of chemically linked monomer repeat units joined at the ends to form a ring, e.g., cyclodextrin (strictly a cyclic oligomer).
Dendritic polymer – a highly structured spherical polymer grown in successive generations from a central “core” moiety, in which every monomer in a generation contains a branch point and allows the attachment of more than one repeat unit in the next generation, e.g., poly(propylene imine).
Graft copolymer – a branched polymer in which the branches have a different chemistry than the main chain, e.g., poly(L-lysine)-g-poly(ethyleneoxide).
Homopolymer – a polymer made of only one type of monomer repeat unit, e.g., poly(methyl methacrylate).
Linear polymer – a polymer that is a single continuous chain of chemically linked monomer repeat units without any branching, e.g., polystyrene.
Monomer – a molecule that can be polymerized to form a polymer.
Oligomer – a small macromolecule consisting of around 10 to 100 chemically bonded monomer repeat units.
Polymer – a macromolecule consisting of at least around 100 chemically bonded repeat units. Can have more than one type of repeat unit.
Random copolymer – a polymer comprising more than one type of repeat unit, chemically linked in as arbitrary sequence.
Star polymer – a polymer in which three or more linear polymer chains radiate from a central, multifunctional moiety to which they are all chemically attached, either by coupling or by being grown from that moiety in the polymerization step.
Terpolymer – a copolymer comprising three different monomer repeat units, e.g., poly(acrylonitrile-co-butadiene-co-styrene).
Chemistry
Chain growth (addition) polymerization – a polymerization occurring by addition of successive monomer units, one at a time, to a reactive growing polymer chain, e.g., free-radical polymerization, ring-opening polymerization, ionic polymerization.
Dispersion polymerization – a polymerization carried out initially in a one-phase system with monomer, polymer, and initiator dissolved in a common solvent in which the polymer is insoluble. The polymer precipitates during the reaction to yield a dispersion of polymer particles in the liquid.
Emulsion polymerization – a polymerization performed in an oil-in-water emulsion (monomer in the oil droplets, surfactant and initiator in the aqueous continuous phase) using a water-soluble initiator where initiation occurs in the aqueous continuous phase. The polymerization occurs mainly in the monomer-swollen surfactant micelles in the continuous phase, fueled by diffusion of monomer from the droplets to the micelles, and not in the monomer droplets themselves. The result is a polymer latex; a dispersion of polymer particles usually less than 1 μm in diameter with a narrow size distribution.
Initiation – the first step in a chain growth polymerization in which an active species reacts with the first monomer of the polymer chain.
Initiator – a molecule that can react with a monomer molecule to start a chain growth polymerization. Usually a free-radical generator (e.g., peroxide, peroxydicarbonate, azo-compound) or, in some cases, a carbon-centered cation (e.g., (CH3)3C+ AlCl3(OH)-) or anion (e.g., C4H – Li+).
Polymerization – a chemical reaction that converts monomer molecules to macromolecules.
Living polymerization – a chain growth polymerization in which the active end of the growing polymer chain is stabilized chemically during the reaction so that termination is controlled, allowing polymer architecture and molecule weight distribution to be a precisely controlled. Anionic and cationic polymerizations are usually living polymerizations. Stabilized free-radical polymerization (SFRP) is a living free-radical-initiated chain growth polymerization.
Propagation – the chemical reaction of monomer units to incorporate them into a growing chain.
Solution polymerization – a polymerization carried out in a one-phase system with monomer, polymer, and initiator dissolved in a common solvent, in which the final polymer product is also soluble.
Step growth (condensation) polymerization – a polymerization that proceeds by linking together multi-functional monomers to first dimers (two monomers) then trimers (three monomers), etc., which themselves link together to form a high molecular weight polymer towards the end of the polymerization, e.g., polyesterification, polyamidation.
Suspension polymerization – a polymerization performed in an oil-in-water emulsion using an oil-soluble initiator (monomer and initiator form the oil droplets, surfactant is in the aqueous phase). The polymer is formed in the emulsified oil droplets and the resulting size distribution reflects that of the starting emulsion droplet size distribution.
Termination – the chemical reaction that ends the growth of a polymer chain.
 Chemical Classification
Chemical Classification
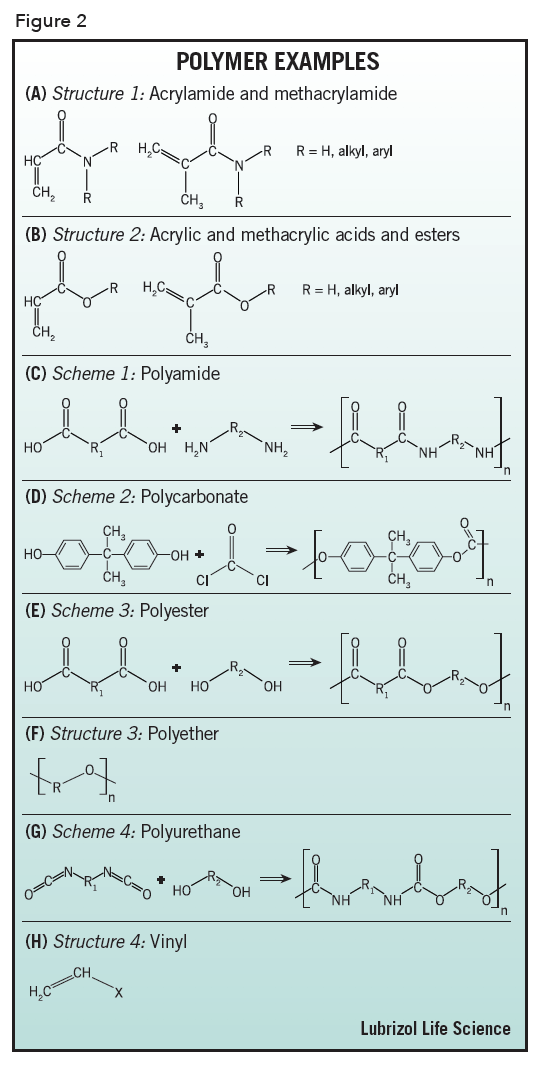
Acrylamide – a polymer formed from monomers based on acrylic acid amides or methacrylic acid amides, see Figure 2 (A), e.g., polyacrylamide.
Acrylic – a polymer formed from monomers based on acrylic acid and its esters or methacrylic acid and its esters, see Figure 2 (B), e.g., Poly(acrylic acid) (Carbopol®, Carbomer), poly(methyl methacrylate-co-methacrylic acid) (Eudragit™).
DNA – macromolecule carrying biological information made from chemically linked nucleotides, in which the sugar is deoxyribose and the bases are adenine, guanine, thymine, and cytosine.
Oligonucleotide – a cellular oligomer, usually less than fifty repeat units long, made from chemically linked nucleotides.
Polyamide – a polymer formed by reaction of poly(carboxylic acids) with polyamines, whose monomer repeat units are linked together by amide groups, see Figure 2 (C), e.g., poly(hexamethylene adipamide) (Nylon 6, 6).
Polycarbonate – a polymer whose monomer repeat units are linked together by carbonate groups, most commonly by reaction of bisphenol-A with phosgene, see Figure 2 (D), e.g., Lexan™.
Polyester – a polymer formed by reaction of poly(carboxylic acids) with polyols, whose monomer repeat units are linked together by ester groups, see Figure 2 (E), e.g., polyethylene terephthalate (PET, e.g. Mylar®), polycaprolactone, poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA).
Polyether – a polymer whose monomer repeat units are linked together by ether linkages, see Figure 2 (F), e.g., poly(ethylene glycol) PEG.
Polysaccharide – naturally occurring polymers from a living source formed by sugar molecules linked together by glycosidic bonds. Semi-synthetic polysaccharides are formed by chemical modification of naturally occurring polymers, e.g., cellulose, chitin, starch, glycogen, xanthan gum, acacia gum, locust bean gum, carrageenan, alginate, chitosan, ethylcellulose, methyl cellulose, hydroxyethyl cellulose (HEC), hydroxypropyl methylcellulose (hypromellose, HPMC), cellulose acetate phthalate (CAP).
Polyurethane – a polymer formed by reaction of polyisocyanates with polyols, whose monomer repeat units are linked together by urethane (carbamate) groups, see Figure 2 (G).
Protein – a wide variety of naturally occurring polyamides made from amino acid monomers, which are used in a multitude of biological processes (signaling, catalysis, structure-formation, etc.).
RNA – macromolecule carrying biological information made from chemically linked nucleotides, each made of a nitrogenous base (adenine, guanine, uracil, or cytosine), the sugar ribose, and one to three phosphate groups.
Vinyl – a polymer made by polymerizing monomers that contain the vinyl group, see Figure 2 (H), e.g., poly(vinyl pyrrolidinone) (PVP), poly(vinyl alcohol) (PVA), EVA, polytetrafluoroethylene (PTFE, Teflon™).
Physical Properties
Glass transition temperature (Tg) – the temperature below which a polymer originally in the amorphous, rubbery state moves to a brittle, amorphous glassy state. The transition from rubber to glass (or glass to rubber) is a second-order transition. Polymer chains can move past each other above Tg, making the bulk polymer processable.
Melting temperature (Tm) – a temperature at which crystalline or semi-crystalline polymers melt to form an amorphous phase. Tm is higher than Tg.
Thermoplastic – a polymer that undergoes reversible softening upon heating and can be molded when hot.
Thermoset – a polymer that chemically cures (crosslinks) upon heating into a form that does not soften on reheating.
Example Uses
Containers – polyethylene, poly- propylene, polycarbonate, polystyrene, EVA.
Dispersion / emulsion stabilizers
– PVA, PEG, PVP, chitosan, proteins, poly(ethylene-co-maleic an- hydride), Pluronic.
Drug-loaded controlled-release particles – PLA, PLGA, PVP, CAP.
Enteric coatings – Eudragits, CAP, cellulose acetate succinate, sodium alginate, hydroxypropyl methylcellulose phthalate, polyvinyl acetate phthalate.
Gene transfer agents – poly(ethylene imine) (PEI), chitosan.
Medical devices – EVA, PTFE, polyurethanes.
Sterile filter membranes – PTFE, nylon, cellulose.
Thickeners (rheology modifier, gel-former) – Carbopol, Carbomer, HEC, HPMC, xanthan gum, carrageenan, PEG, PVA, chitosan, acrylamide.