
Cubic Phase Particles
Delivery of active pharmaceutical ingredients (APIs) has taken center stage over the last decade. Focused initially on problematic new chemical entities (NCEs), innovative delivery systems are now the go-to approach for both NCE commercialization and the refinement and repurposing of existing APIs. The needs generally fall into one or more categories listed in Table 1. Of course, impacting any one of these properties will likely impact some or all of the others. In addition to the physicochemical and physiologic goals listed in Table 1, business concerns also drive the use of sophisticated delivery systems. Intellectual property, life-cycle extension, cost, and market differentiation are examples.
 While these may seem daunting, there are common fundamental properties that drive real-world possibilities. Compounding the difficulty in establishing a decision tree is the fact that there is a myriad of drug delivery approaches being touted by industry players. However, there are a limited number of technologies that are commercially viable and have the characteristics outlined in Table 2. If one is developing a drug product with the intent of marketing it in the foreseeable future, say 10 years or less, success is much more likely when utilizing an approach that meets the criteria in Table 2. In the scenario where the technology is compelling, but one or more Table 2 criteria are not met, one should have a solid plan to address the deficiency.
While these may seem daunting, there are common fundamental properties that drive real-world possibilities. Compounding the difficulty in establishing a decision tree is the fact that there is a myriad of drug delivery approaches being touted by industry players. However, there are a limited number of technologies that are commercially viable and have the characteristics outlined in Table 2. If one is developing a drug product with the intent of marketing it in the foreseeable future, say 10 years or less, success is much more likely when utilizing an approach that meets the criteria in Table 2. In the scenario where the technology is compelling, but one or more Table 2 criteria are not met, one should have a solid plan to address the deficiency.
Reversed cubic phase liquid crystal dispersion (LyoCell®) is an example of a technology that meets the criteria in Table 2 and can have a significant impact on the properties outlined in Table 1. Liquid crystals are compositions that have properties of both liquids and crystals. For example, molecules in liquid crystals have an ordered orientation but the composition as a whole may flow. There are several types of liquid crystals, lyotropic being one of them. Within lyotropic liquid crystals, the reversed cubic phase structure is a specific sub-category with unique properties.
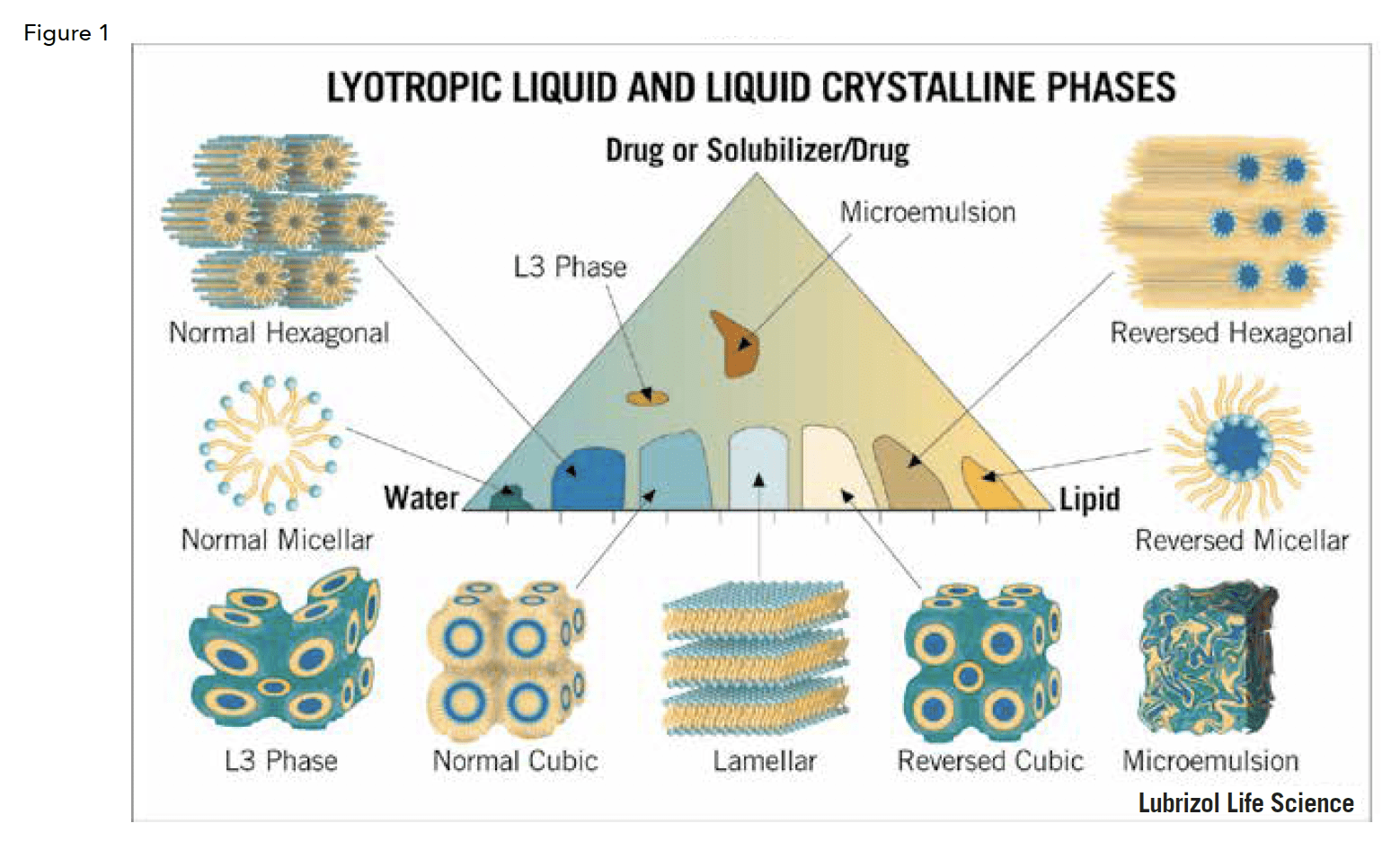
 As can be seen in Figure 1, lipids in the presence of water and, optionally, a solubilizer, can form various structures along a continuum and are dictated by well-documented physical rules. The phase diagram in Figure 1 illustrates the relative compositions of the various lyotropic liquid crystalline phases and their structures. Note that this phase diagram is for a specific system, as each system is different. A full discussion on the properties of each of these phases and the thermodynamic basis for their existence is well beyond the scope of this review. Therefore, we will just briefly say that careful mapping and data-driven application of phase behavior can yield predictable and highly reproducible results. Further, properties within a phase can be manipulated to meet various needs.
As can be seen in Figure 1, lipids in the presence of water and, optionally, a solubilizer, can form various structures along a continuum and are dictated by well-documented physical rules. The phase diagram in Figure 1 illustrates the relative compositions of the various lyotropic liquid crystalline phases and their structures. Note that this phase diagram is for a specific system, as each system is different. A full discussion on the properties of each of these phases and the thermodynamic basis for their existence is well beyond the scope of this review. Therefore, we will just briefly say that careful mapping and data-driven application of phase behavior can yield predictable and highly reproducible results. Further, properties within a phase can be manipulated to meet various needs.
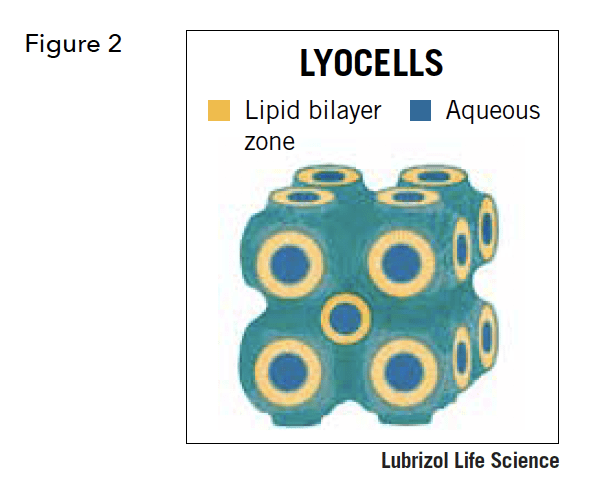
 From a pharmaceutical perspective, several properties of reversed cubic lyotropic liquid crystals are particularly attractive. As seen in Figure 2, a lipid bilayer forms the non-aqueous portion of the structure. A key component of this lipid layer is that it is continuous throughout the entire mass of the reverse cubic phase. Likewise, so is the aqueous or polar portion. In contrast to a liposome, or emulsion, or other structured lipid phases, a reversed cubic phase lyotropic liquid has no inner or outer surface. It is characterized by its ”bicontinuity”, meaning that both the lipid and polar components are continuous and interpenetrating.
From a pharmaceutical perspective, several properties of reversed cubic lyotropic liquid crystals are particularly attractive. As seen in Figure 2, a lipid bilayer forms the non-aqueous portion of the structure. A key component of this lipid layer is that it is continuous throughout the entire mass of the reverse cubic phase. Likewise, so is the aqueous or polar portion. In contrast to a liposome, or emulsion, or other structured lipid phases, a reversed cubic phase lyotropic liquid has no inner or outer surface. It is characterized by its ”bicontinuity”, meaning that both the lipid and polar components are continuous and interpenetrating.
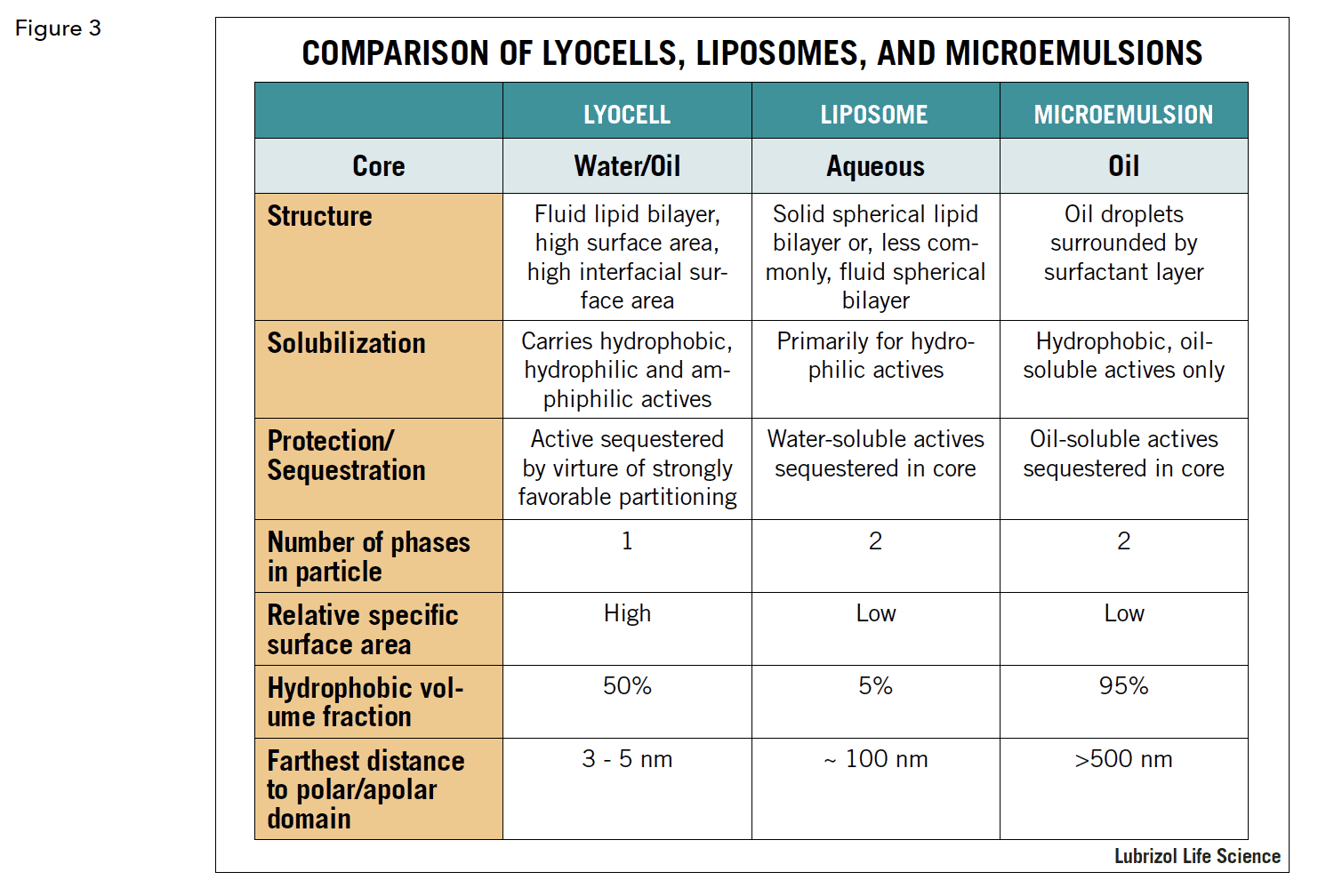
 A result of this is that polar and non-polar domains are always close to one another, on the order of 3 to 5 nanometers, imparting very powerful solubilization properties to reversed cubic phase lyotropic liquid crystals. This is in contrast to liposomes, for instance, where the lipid layer is a shell to an aqueous core, or to standard emulsions, where an oil core is surrounded by a surfactant layer. Figure 3 compares the three constructs. One result of this is that reversed cubic lyotropic liquid crystals can be loaded with a multitude of APIs with varying charges and partition coefficients. Another result of their unique structure is that reversed cubic liquid crystals are highly compatible with biologic membranes, at least partially accounting for their unique drug delivery properties. While the math behind this is complex, the basic principle is that, not only does the chemical similarity promote compatibility between the cubic phase and biologic membranes, but so does the actual shape, specifically, the negative curvature. Cubic phase materials are also very adhesive, making them ideal for use, in the bulk form, for applications such as mucosal or topical delivery.
A result of this is that polar and non-polar domains are always close to one another, on the order of 3 to 5 nanometers, imparting very powerful solubilization properties to reversed cubic phase lyotropic liquid crystals. This is in contrast to liposomes, for instance, where the lipid layer is a shell to an aqueous core, or to standard emulsions, where an oil core is surrounded by a surfactant layer. Figure 3 compares the three constructs. One result of this is that reversed cubic lyotropic liquid crystals can be loaded with a multitude of APIs with varying charges and partition coefficients. Another result of their unique structure is that reversed cubic liquid crystals are highly compatible with biologic membranes, at least partially accounting for their unique drug delivery properties. While the math behind this is complex, the basic principle is that, not only does the chemical similarity promote compatibility between the cubic phase and biologic membranes, but so does the actual shape, specifically, the negative curvature. Cubic phase materials are also very adhesive, making them ideal for use, in the bulk form, for applications such as mucosal or topical delivery.
 Reversed cubic lyotropic liquid crystals are formed by self-assembly in accordance with the laws of thermodynamic phase equilibria. In bulk, reversed cubic liquid crystals are highly viscous, optically anisotropic (non-birefingent) semisolids. The bulk can be dispersed to form particles in a range of sizes, including those in the nanometer range. When dispersed, each particle is referred to as a LyoCell. Each LyoCell retains the superstructure of the bulk reversed cubic phase. A 200 nm LyoCell, for instance, will consist of approximately one 10 x 10 block of the individual units. Figure 2 shows a 2 x 2 block by way of example. The pores or channels in a LyoCell are ~10 nm in diameter. LyoCells feature lipid:water ratios typically on the order of 1:1, a fluid bilayer, and a nanostructure in which every point in the cubic phase lies near both a polar and an apolar domain. The extended interfacial area (~200 m2/g) provides a richly amphiphilic milieu for effective solubilization of most compounds of interest in pharmaceuticals, from small molecules, like paclitaxel, to proteins.
Reversed cubic lyotropic liquid crystals are formed by self-assembly in accordance with the laws of thermodynamic phase equilibria. In bulk, reversed cubic liquid crystals are highly viscous, optically anisotropic (non-birefingent) semisolids. The bulk can be dispersed to form particles in a range of sizes, including those in the nanometer range. When dispersed, each particle is referred to as a LyoCell. Each LyoCell retains the superstructure of the bulk reversed cubic phase. A 200 nm LyoCell, for instance, will consist of approximately one 10 x 10 block of the individual units. Figure 2 shows a 2 x 2 block by way of example. The pores or channels in a LyoCell are ~10 nm in diameter. LyoCells feature lipid:water ratios typically on the order of 1:1, a fluid bilayer, and a nanostructure in which every point in the cubic phase lies near both a polar and an apolar domain. The extended interfacial area (~200 m2/g) provides a richly amphiphilic milieu for effective solubilization of most compounds of interest in pharmaceuticals, from small molecules, like paclitaxel, to proteins.
Production
Reversed cubic phase lyotropic liquid crystals and their dispersed form, LyoCells, are easily manufactured using standard techniques and FDA generally recognized as safe (GRAS) excipients. Acceptable components exist for all dosage forms, including parenterals. Going back to the phase diagram, there are several paths that will lead to the right structure. Which one is best is based on experience and careful consideration of the API and the Target Product Profile. As an example, the non-aqueous components can be mixed with the API, often at room temperature. This results in a non-structured mixture. As water or another polar solvent is added, the reversed cubic phase spontaneously forms. In practice, this transition can be followed and controlled to meet specific needs. Once formed, the reversed cubic phase is a high viscosity, well-ordered system. The drug is found in equilibrium situated in the LyoCell as dictated by its solubility. For instance, a hydrophobic small molecule will be found intercalated within the lipid later on. Amphiphilic molecules will bridge the lipid/polar interface as would a membrane protein.
To form LyoCells, the bulk cubic phase is dispersed into water or another polar liquid. As above, the bulk breaks down into smaller, yet intact, particles that retain the same physicochemical properties. Stabilizers added to the aqueous phase are used to achieve smaller particle sizes – in the nanometer range.
LyoCells are generally stable to irradiation and, when dispersed to the proper size, are sterile filterable. They can have a negative or positive surface charge depending on the application and can even be encapsulated, adding another dimension of control to API release. Because of the excipients and the fact that they are continuous with the polar phase, LyoCells have a neutral density and tend not to settle or cream when in suspension. Lastly, LyoCells can be converted to a powder if needed.
In summary, reversed cubic phase materials offer an excellent choice for high impact formulation needs. In addition, they meet the requirements of a commercially viable technology. Several products are already in development using LyoCells and that number promises to rapidly increase.