Process Analytical Technology
Table of Contents
Process analytical technology (PAT) can be viewed from many perspectives: from mathematical to chemical to regulatory or from production worker to manufacturing engineer. This technical brief focuses from a high-level perspective.
Background
PAT has grown over the past several decades, driven by the need for improving manufacturing productivity across all industries1. In the highly regulated pharmaceutical sector, advances are accompanied by worldwide regulatory initiatives, such as the FDA guidance documents on Good Manufacturing Practices, PAT, and Quality by Design (QbD), which have influenced agencies, including the International Conference on Harmonization 2-4 . This paradigm shift of regulatory agencies has promoted the QbD approach for future product submissions and has changed the way companies do business.
Definition of PAT
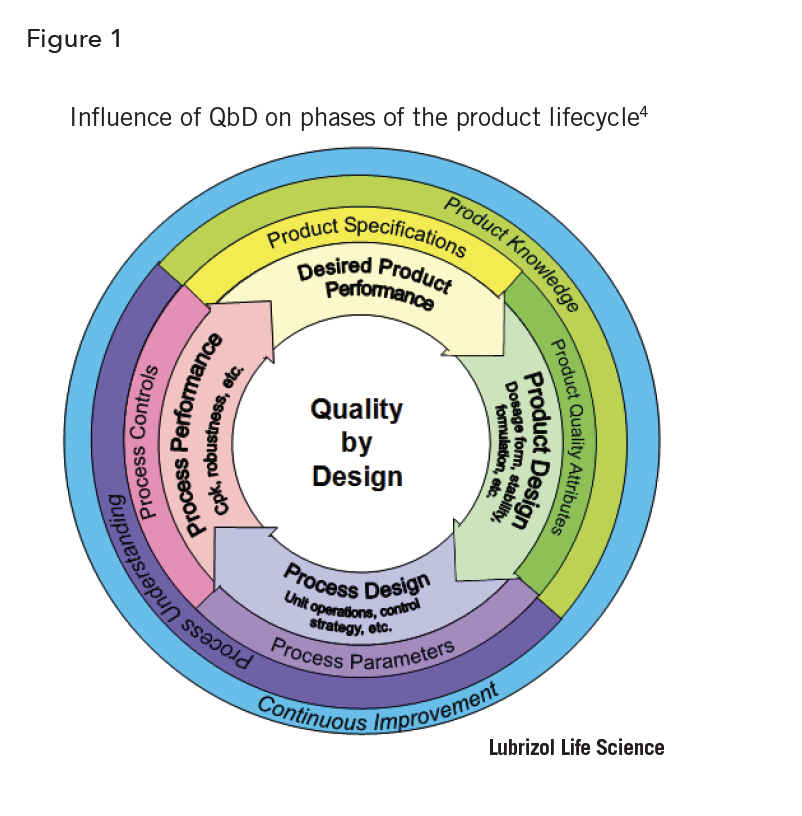
PAT is used to describe a change in pharmaceutical manufacturing from static batch manufacturing to a more dynamic approach. By definition, PAT is the deployment of instrumentation for real-time, continuous analysis of manufacturing processes and involves use of mathematical modeling (chemometrics) for monitoring chemical and/or physical critical quality attributes (CQAs). PAT is a supplemental technique that allows for detection of events that cannot be derived from conventional discrete variables such as temperature, pressure, and flow rate. Effective implementation involves the use of different technologies to support a science-based understanding of the physical, chemical, and mechanical properties of the various manufacturing processes. PAT is a testing and feedback loop which allows for process adjustment based upon thorough knowledge of how the components and related processes affect the final product. This is in accordance with the fundamental principle that quality cannot be tested but should rather be built into the product by design3. Manufacturing QbD in part involves PAT strategies to reduce identified manufacturing risks that are associated with product quality. All these factors are reflected in product development and process design is subject to continual improvements throughout the lifecycle of the product (Figure 1) 4.
Goal of PAT
The goal of PAT is to design and develop dynamic manufacturing processes that can compensate for variability in both raw materials and equipment, in order to consistently ensure a predefined quality at the end of the process. The central objective is to generate product quality information in real-time, so current and downstream operations to be adjusted accordingly. PAT aims to ensure that all sources of variability affecting a process are identified, explained and managed.
Industry Driver
The growing use of more complex PAT within manufacturing industries is driven by increased technical capabilities that provide improved engineering controls. Historically, due do the stringent regulatory nature of the industry, the PAT initiative in the traditional pharmaceutical sector has lagged behind that of other industries.
With the more recent FDA directives, pharmaceutical companies have begun to change the framework in which they develop, implement, monitor, and control their manufacturing processes. Learning from other industries, pharmaceutical companies now realize that if a process can be monitored at critical points and the information can be used to actively control the process, then a consistent, reliable, lower cost, and high-quality final product results. Drivers to implement PAT include the opportunity for live feedback and process control, cycle time reduction, and laboratory test replacement as well as risk mitigation. With such advances and knowledge of the product design space, there is the possibility of real-time-release as well as less scale-up and post-approval challenges for process or production site changes. PAT also carries the promise of new and innovative methods of production, as continuous monitoring allows for more controlled processes and better understanding and control of intermediate steps.
How PAT works
To successfully implement PAT, a combination of sequential steps is essential.
First, a unit operation or process must be defined as requiring PAT or being amenable to PAT deployment. In many cases, a thorough lab-scale feasibility evaluation of analytical methods is conducted to determine which techniques may have adequate sensitivity and selectivity. Additionally, PAT goes through extensive cost-benefit analysis as to which approach may reach the production floor.
Second, a suitable PAT technique needs to be chosen which would allow for measurement of the critical process parameter (CPP), preferably in an in-line or at-line manner. The first step away from offline laboratory testing would be at-line testing, which moves process dedicated testing equipment to the production line. One approach of PAT is online testing, which draws samples and monitors periodically. Another mode is inline testing, which places probes in constant contact with the drug product. The advantage of on- or in-line testing is better control, because the analysis provides the most up-to-date snapshot of the process. Near infrared (NIR) spectroscopy is one of the most routinely used techniques and has gained wide acceptance. NIR is a rapid, nondestructive technique that eliminates the need for sample preparation and enables real-time monitoring.
Next, logistical constraints would need to be considered, like how to interface the PAT technique with the process, such as whether the equipment had applicable NIR fiber optic probe-interface ports or if modifications were necessary.
Finally, the PAT method would need a reference method for correlation of spectral data versus a traditional method value. In many instances, such as inline content uniformity, high-performance liquid chromatography (HPLC) data is correlated to NIR spectral data to build an accurate PAT model.
In order to generate a spectral model, chemometric software is utilized to analyze spectral data in comparison to known reference values. The PAT technique and associated models are continually updated, verified, and validated.
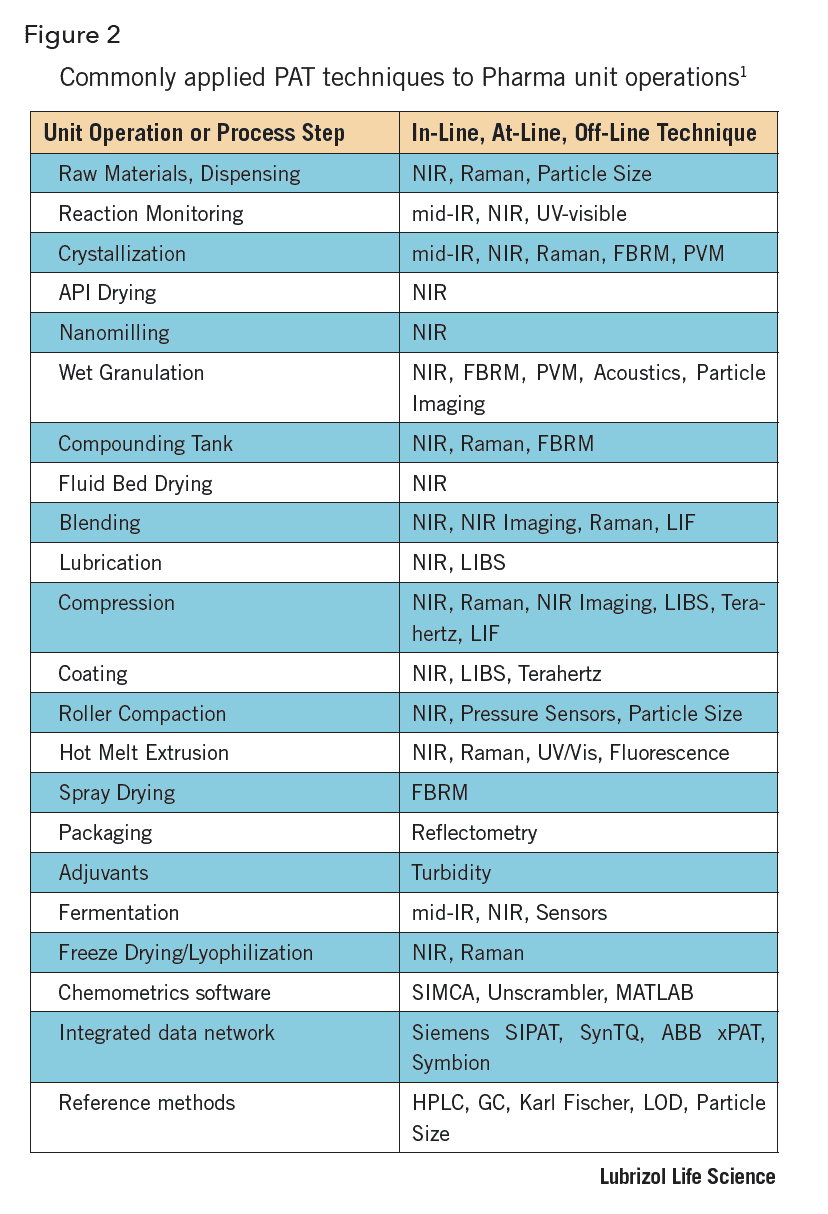
PAT is not limited to NIR and can include many other analytical methods. These are performed in coordination with qualified and validated process analyzer hardware, operating software, and a chemometric data analysis package. The implementation of PAT will vary widely depending on the production technology or unit operation, as well as technical hurdles of the available technology. Figure 2 lists common pharmaceutical unit operations and applied PAT techniques.
 Example: PAT for Hot Melt Extrusion
Example: PAT for Hot Melt Extrusion
Hot Melt Extrusion (HME) is a common manufacturing operation in the food and polymer industries and is becoming increasingly deployed in the pharmaceutical industry. Due to the continuous, efficient nature of HME, which combines several polymer drug mixing and processing steps, it is often used to manufacture large, expensive batches of extruded material. Therefore, it is imperative that product composition is monitored, and constancy ensured throughout the batch. Traditional offline techniques, such as chromatography, can be used for these compositional measurements, but the nature of the polymer extrudate and drug extraction makes sample preparation difficult and untimely. When applied to HME, NIR eliminates the need for sample preparation and time-consuming offline analyses.
Aside from content uniformity, NIR can be used for extruder performance verification (residence time distribution studies, process startup, feeder refill fluctuations, etc.). NIR or Raman can be used for assessing polymer layer thickness in multi-polymer systems. Additionally, when the extrusion process is interfaced with Raman, the crystal-form of the drug can be monitored.
PAT Strategy during Stages of Development
Several types of PAT applications can accompany the lifespan of a product or process. The drivers, challenges, benefits, and organizational support considerations, as well as cost associated with these types of methods, can be very different from R&D through full-scale production. Pharmaceutical, generic, and contract manufacturing organizations span the gamut from employing little to no PAT implementation to having corporate supported platform approaches for unit operations in commercial/supply operations. During early phase R&D process optimization, a broad range of PAT methods are used by a subset of collaborative project team members to gain chemical and physical understanding of the unit operation specifics for a particular formulation. The scale-up phase broadens the goal of PAT to continue to gain process knowledge as well as to verify the scalability of the process by comparing process data obtained during initial development with pilot plant or full-scale data sets at various manufacturing sites. Finally, PAT methods that are scientifically- and commercially-justified are utilized either as temporary methods for gaining process information, troubleshooting, or as permanent installations as part of the validated process. Continual deployment of PAT across R&D through commercial stages will enable new products to move into the market faster and more easily.
Pharmaceutical companies will develop different approaches to exploit PAT, which can range from using the initiative in a tactical manner to fix poorly performing or misunderstood processes and/or to move to a right-first-time QbD and business strategy. To support future QbD product filings, the comprehensive PAT strategy will require a wide set of scientific and measurement knowledge, as well as management and process and application integration capabilities.
Conclusions
PAT offers the pharmaceutical industry a framework for revolutionizing its R&D and manufacturing businesses, therefore producing value for themselves and, ultimately, the patient and consumer.
References
- Bakeev K, Process Analytical Technology: Spectroscopic Tools and Implementation Strategies for the Chemical and Pharmaceutical Industries, Wiley,
- Pharmaceutical cGMPs for the 21st Century: A Risk-Based Approach, FDA, Fall
- Guidance for Industry: PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance, FDA, September
- A New Pharmaceutical Quality Assessment System for the 21st Century, FDA, December