A Pharmaceutical Guide to Cannabis – FAQs for Drug Product Development
Table of Contents
Questions about the regulations and legal status of cannabidiol (CBD) generate different, and often convoluted, answers depending on who is asked. Despite the growing availability of CBD, particularly since the 2018 Farm Bill was signed into law, a number of factors remain unclear, including:
- Federal and state regulatory oversight of CBD manufacturing
- Use of CBD as an active pharmaceutical ingredient (API) in drug products
- Use of CBD in over-the-counter nutraceutical formulations
- Marketing of CBD products
In this interview, Judy Cohen, Vice President of Quality and Regulatory for the Particle Sciences, addresses a number of questions regarding CBD regulations with emphasis on its use in the pharmaceutical space. We begin with an overview of Cannabis extraction and its use in drug products to date.
Overview of CBD Terminology
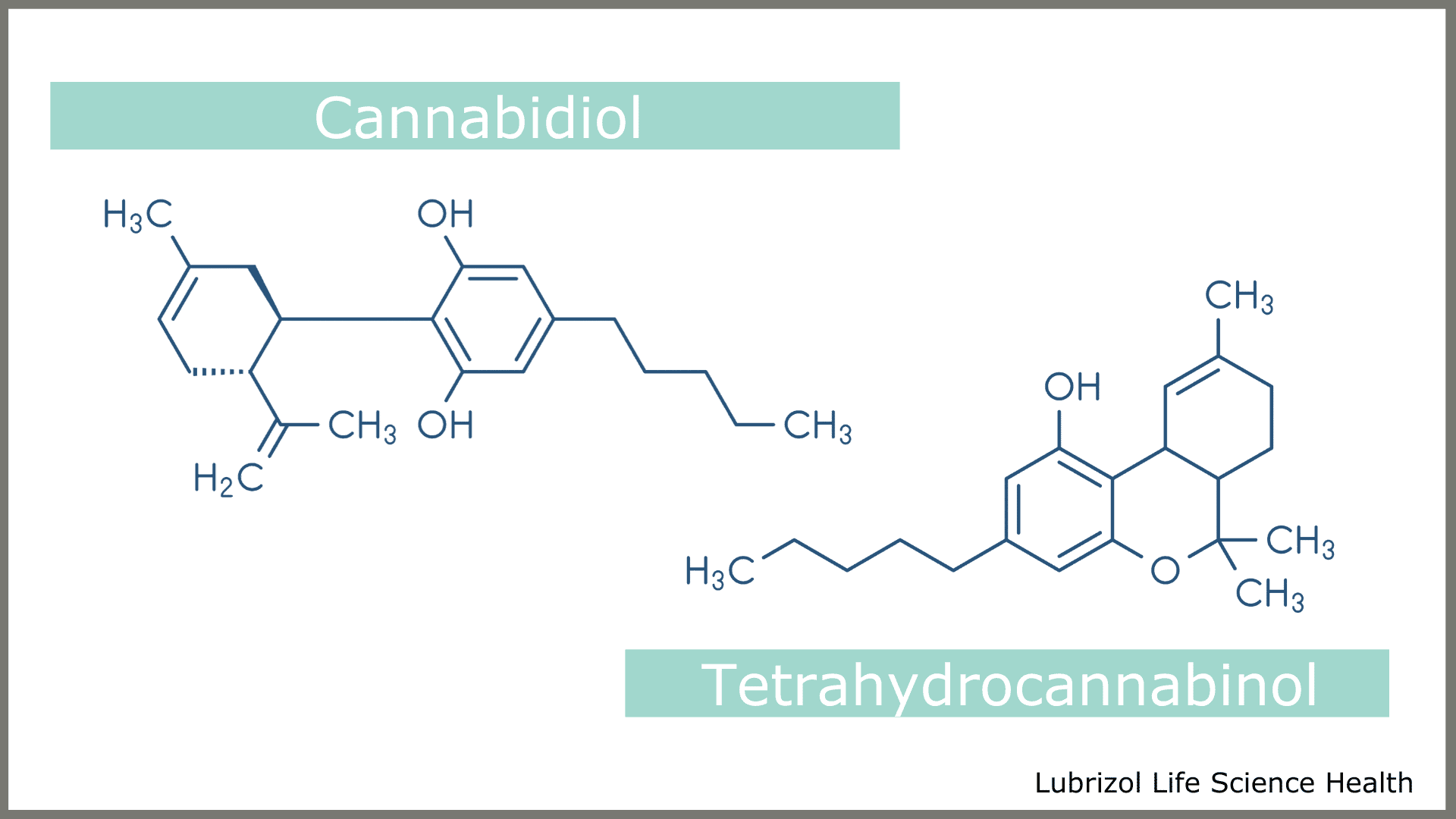
Cannabis is the genus of the plant from the Cannabaceae family. There are three main species of Cannabis: C. sativa L., C. indica, and C. ruderalis. C. sativa L. is the most well-known species and commonly used to extract CBD for many uses and applications. There are also two main types of C. sativa L., hemp, and what is referred to as marijuana. Although CBD is found in both hemp and marijuana, the main difference between these two forms is that hemp contains almost none of the psychotropic compound that causes the “high” found in marijuana. The psychotropic compound of marijuana (tetrahydrocannabinol or THC) and CBD are in the chemical class termed cannabinoids (Figure 1).
Types of CBD
The Cannabis plant has well over 400 different compounds, and 15-20% of these are cannabinoids. Members of the classic cannabinoid chemical class are phenolic compounds that mimic naturally occurring endocannabinoids in the body. Cannabinoids interact in different ways with CB1 and CB2 receptors found throughout the body, including the brain. There is emerging evidence that CBD may also act on targets other than endocannabinoid receptors.
Figure 1: Chemical Structures of CBD and THC
The Agriculture Improvement Act of 2018 (the 2018 Farm Bill), was signed into law on December 20, 2018. The new law affects the status of the entire hemp plant and derivatives, including how it is produced and marketed. The legislation dictates that hemp and its corresponding products must have a THC content of 0.3% or less. As a result of this restriction, hemp and products derived from it are not subject to the Controlled Substances Act, which places regulations on the manufacture, importation, possession, use, and distribution of certain substances with high abuse potential.
Cannabis and the Pharmaceutical Industry
Drug products containing a synthetic form of THC (dronabinol) have been approved by the U.S. FDA (FDA) for decades (Table 1). Epidiolex, a seizure treatment approved in 2018, was the first Cannabis-derived CBD drug to be approved in the U.S.
Table 1: Cannabinoid Drugs Approved by the FDA
| Drug Product | Year Approved | API | Indication(s) |
| Marinol | 1985 | Dronabinol | Cancer – prevent nausea, vomiting, inappetence |
| Sativex | 2010 (UK) | 1:1 Ratio THC:CBD | Multiple sclerosis – pain |
| Nabiximols
(Sativex) |
FDA approval pending | C. sativa L. extract | Schizophrenia and neurological conditions |
| Syndros | 2017 | Dronabinol | Anorexia – AID syndrome
Cancer – nausea and vomiting |
| Epidiolex | 2018 | CBD | Lennox-Gastaut syndrome/Dravet syndrome – seizures |
There are other indications of the pharmaceutical industry’s growing interest in developing Cannabis-based products, including the agreement between Tilray (a Canadian Cannabis company) and Sandoz, a subsidiary of Novartis. The objective of this partnership is for existing and future medical marijuana products to be sold in any of the 35 countries where medical marijuana is legal. Another start-up pharmaceutical company, Pure Green Pharmaceuticals, Inc., patented a soluble sublingual tablet containing CBD and THC that can be rapidly absorbed into the circulatory system (comparable to smoking Cannabis). The tablets are undergoing clinical trials to determine their effectiveness in treating osteoarthritis and other musculoskeletal pain. The company has another tablet containing CBD and THC that is in clinical trials for menstrual pain relief.
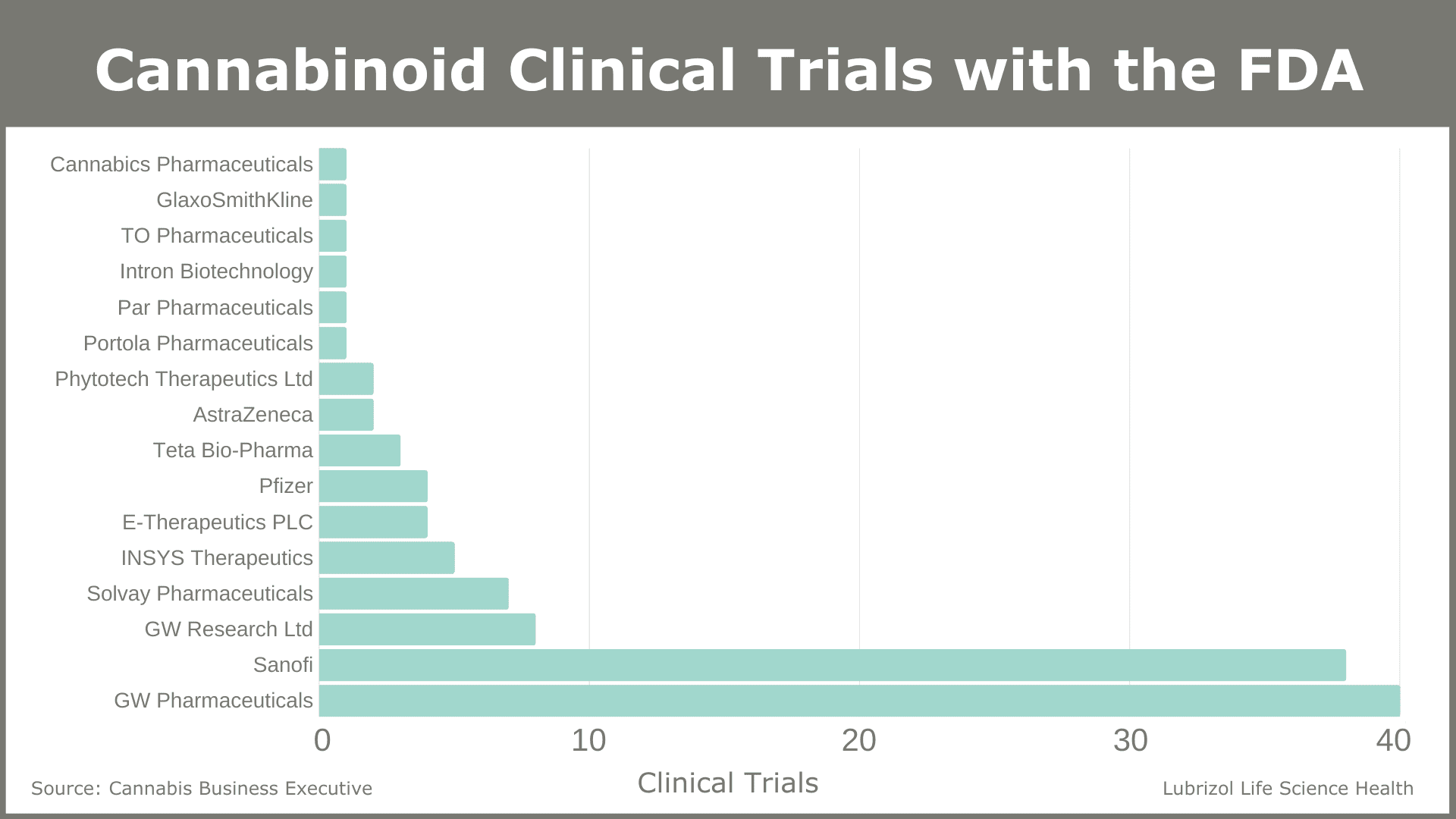
According to data compiled and provided by Cannabis Business Executive, there were 16 pharmaceutical companies conducting cannabinoid clinical trials as of June 2018. Figure 2 shows the number of clinical trials for each company.
Figure 2: Companies with Clinical Trials Registered with the FDA
The market availability of CBD overall is widespread and ever-growing. However, due to manufacturing inefficiencies and poor-quality control/oversight, many products do not meet their label claim for CBD content. The pharmaceutical arena is poised to offer CBD products with consistent quality that builds consumer confidence. With strict, GMP manufacturing standards already in place, pharmaceutical companies and contract development and manufacturing organizations (CDMOs) can produce higher quality products than their consumer counterparts. Clinical studies to determine the efficacy of CBD for several conditions provide an opportunity for pharmaceutical companies to add another portfolio of treatments to their current pipelines.
Frequently Asked Questions About CBD with Judy Cohen
In the following interview, Judy Cohen addresses questions regarding the regulatory issues surrounding CBD. Of particular interest is the scheduling and production regulations that apply to the provision of CBD-related materials for use in pharmaceutical drug products.
Q: What changes have occurred with regards to CBD regulation?
A: The Cannabis plant known as marijuana is still categorized as a Schedule I substance (the strictest classification under the Controlled Substances Act). Substances in this category are considered by the U.S. Drug Enforcement Administration (DEA) to have no accepted medical use and a high potential for abuse. Hemp was previously in this category, but this changed when the 2018 Farm Bill became law, provided that the hemp and derived products contain less than 0.3% THC. The challenge to this bill was ensuring that hemp contained less than 0.3% THC, and further, the transfer of this material over state lines wasn’t well understood. The United States Department of Agriculture (USDA) in October 2019 published its interim final rules for domestic hemp production in the Federal Register, including rules regarding the testing of hemp in DEA-registered testing sites. This has provided clarity to the original 2018 Farm Bill.
Q: The FDA has categorized CBD as a drug, will this lead to the scheduling of CBD?
A: The drug Epidiolex was approved in 2018 to treat seizures in patients with forms of epilepsy. Epidiolex was the first cannabis-derived drug product to obtain FDA approval, and it was originally classified as a Schedule I controlled substance. Months after its approval, the DEA reclassified Epidiolex—and future drugs containing CBD with no more than 0.1 % THC—as Schedule V controlled substances (the least strict classification under the Controlled Substance Act). In April of 2020, the DEA took the final step and descheduled Epidiolex, removing it from the controlled substances list altogether. Currently, Epidiolex remains the only FDA-approved, cannabis-derived drug product on the market, so it is not certain if further drugs with CBD as an active compound will be descheduled.
In July of 2018, the World Health Organization (WHO) Expert Committee on Drug Dependence (ECDD) recommended to the United Nations Commission on Narcotic Drugs (CND) that preparations considered to be pure CBD should be descheduled under any of the international drug control treaties. The WHO also recommended that the scheduling of cannabis and cannabis-related substances reflect their use in pharmaceutical preparations. A CND vote on the ECDD recommendations is scheduled to be considered in 2020.
Q: Which Cannabis-derived products need to come from providers that have Schedule I registration?
A: There is now a distinction between Cannabis-derived material depending on whether it will have pharmaceutical applications. THC has been shown to have more activity than CBD for some pharmacologic applications; therefore, a supplier of this substance would need Schedule I clearance. This is also the case for CBD formulations that have more than 0.3% THC.
Q: How does someone prove that their raw material product has a certain percentage of THC?
A: Testing laboratories that are DEA and FDA registered can perform potency testing to determine the precise amount of a given cannabinoid in a product. A number of these laboratories are also accredited hemp testing labs. The producers may request documentation from the registered testing laboratories to verify THC content.
Q: Does the application of Good Manufacturing Practices (GMPs) apply to hemp-derived products?
Any CBD product which will be used as part of a finished dosage form for a pharmaceutical application must be prepared as an Active Pharmaceutical Ingredient (API) under the appropriate GMP regulatory requirements. API refers to an active ingredient in a drug product intended to affect bodily function and structure, including the prevention, diagnosis, or treatment of disease. For synthetically derived CBD products, this is straight forward as these products typically have a well-defined impurity profile and can be prepared reproducibly according to the required specifications. For natural hemp production, the control of the impurity profile is more challenging and batch to batch variations must be well understood. Further, the CBD must be isolated by companies that adhere to the appropriate GMP regulatory requirements.
Q: Has Particle Sciences used naturally derived or only synthetic CBD products?
A: Particle Sciences has only used synthetic CBD products. Historically, obtaining hemp-derived pharmaceutical-grade products has been a challenge and the batch-to-batch variability has been difficult to control. This may change as more hemp producers look at preparing the CBD according to the appropriate GMP regulatory requirements.
Q: What does a CDMO such as Particle Sciences require from a supplier of CBD materials?
A: The supplier would need to provide documentation regarding the cannabinoid content, and the CBD must be from a GMP facility. If the THC content of the product is over 0.3%, the facility must be DEA registered with Schedule I clearance.
Q: What trends do you see coming forward regarding the CBD arena?
A: With the ability to have a regulatory path for approval, we will see a lot more companies looking at applications for not just CBD, but also THC. However, the FDA will also get more involved with regulatory issues regarding the production of nutraceuticals and personal care products, particularly as many more of these types of products enter the marketplace. The goal will be to ensure products have acceptable purity and safety to enter commerce.
Q: Is there guidance regarding what applications are more suited to be CBD-based than others?
A: There is no guidance on the best uses for CBD, despite the existence of a plethora of product categories containing CBD. In terms of medical uses, there is increasing research to apply CBD-based therapy, and some type of regulatory control would be needed.
Q: What are your recommendations regarding the procurement of cannabinoid materials for pharmaceutical use?
A: CBD should be obtained from a trusted supplier that is GMP certified. Working with a CDMO with expertise in handling these materials for formulating and developing a pharmaceutical is paramount.
Q: What is the best source of information about hemp-related regulations for pharmaceutical clientele?
It is crucial to keep abreast of FDA publications regarding Cannabis-based products, but it is also important to be aware of one’s state policies (found on official state websites), as well as states where products are planned to be marketed. Searching on USDA websites can provide additional information as this agency becomes more important regarding how hemp is produced and tested for THC levels.
List of Abbreviations
API – Active Pharmaceutical Ingredient
CBD – Cannabidiol
CDMO – Contract Development and Manufacturing Organization
CND – United Nations Commission on Narcotic Drugs
DEA – United States Drug Enforcement Administration
ECDD – Expert Committee on Drug Dependence
FDA – U.S. Food and Drug Administration
GMP – Good Manufacturing Practice
THC – Tetrahydrocannabinol
WHO – World Health Organization
Authors: