
Hot Melt Extrusion: Solubility Enhancement, Controlled Release, and More
Table of Contents
Key Points
- Hot melt extrusion is a process that uses heat and pressure to generate homogenous mixtures of polymer and API.
- Hot melt extrusion is a proven, commercially validated process for formulating poorly water-soluble APIs and creating controlled release drug-eluting systems.
- Hot melt extrusion is a versatile and reliable technology that can generate a wide range of dosage forms in a scalable, reproducible manner.
- Multiple classes of API can be processed via hot melt extrusion, including BCS class II and IV compounds, highly potent APIs, and controlled substances.
- There are multiple formulation and process variables that must be optimized based on the physicochemical properties of the API and the desired properties of the final dosage form.
- Hot melt extrusion requires specialized equipment, experienced personnel, and appropriately designed facilities to properly execute.
What is Hot Melt Extrusion?
Hot melt extrusion (HME) is the process of applying heat and pressure to melt a polymer and force it though an orifice in a continuous process. HME is a common processing technique for polymers that dates back to the 1930s. Today, over half of all plastic products are produced via HME. As the technology has evolved over the years, HME has found use in the healthcare industry, where it is used in a range of applications, including:
- Manufacturing of medical devices
- Mixing of active pharmaceutical ingredients (APIs) with polymers
- Production of drug-eluting systems
In this post, we will explore the uses of HME for pharmaceuticals, the equipment and materials needed for HME, and the reasons why HME is considered a valuable tool in complex drug product development.
Table 1: Advantages and Disadvantages of Hot Melt Extrusion
| Advantages | Disadvantages |
|---|---|
| Continuous, reproducible process | Thermal degradation is possible for sensitive materials |
| Efficient, highly automated | High start-up costs |
| Solvent-free and water-free | Specialized knowledge required |
| Customizable to many dosage forms | |
| Proven technology with FDA approvals | |
| Wide range of polymer and API options, including insoluble and highly potent APIs | |
| Suitable for new chemical entities, product line extensions, 505(b)(2)s, and generics |
FDA-Approved Uses of Hot Melt Extrusion
HME was first introduced to the pharmaceutical industry during the 1970s, when the concept of using a polymeric carrier for an API was explored. Since then, HME has been applied to multiple dosage forms, routes of administration, and APIs, demonstrating its versatility as a processing technique. The acceptance of HME by the pharmaceutical industry has led to the production of pharmaceutical-class equipment and the use of quality-by-design methodology, enabling HME to move from the lab into commercial products. Since 1996, multiple products utilizing HME have obtained FDA approval. Table 2 provides examples of these products.
Table 2: Examples of FDA-Approved Products that Utilize Hot Melt Extrusion
| Brand | API | Dosage Form | Year of FDA Approval |
|---|---|---|---|
| Estring® | Estradiol | Vaginal Ring | 1996 |
| Rezulin®* | Troglitazone | Oral Tablet | 1997 |
| NuvaRing® | Ethinyl estradiol, Etonogestrel |
Vaginal Ring | 2001 |
| Implanon® | Etonogestrel | Subcutaneous Implant | 2001 |
| Femring® | Estradiol acetate | Vaginal Ring | 2003 |
| Kaletra® | Lopinavir, Ritonavir | Oral Tablet | 2005 |
| Nexplanon® | Etonogestrel | Subcutaneous Implant | 2006 |
| Neupro®* | Rotigotine | Transdermal Patch | 2007 |
| Viekira Pak® | Dasabuvir sodium, Ombitasvir, Paritaprevir, Ritonavir |
Oral Tablet | 2014 |
* discontinued
Applications of HME
In general, the goal of pharmaceutical HME is to uniformly disperse an API in a carrier polymer. The two most common reasons for using HME in a pharmaceutical setting are:
- To improve the solubility and bioavailability of an API
- To control the delivery of an API
Improved Solubility and Bioavailability
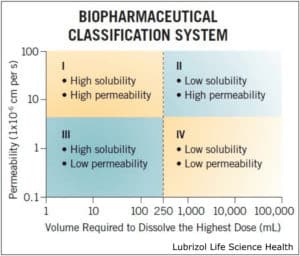
Why It Is Needed: For BCS class II and IV APIs (Figure 1), poor solubility is a direct result of a stable crystalline form that water cannot efficiently penetrate. The amorphous form of an API is often more soluble because less energy is required for dissolution. As a result, solid dispersions were developed to distribute the API in a carrier polymer, preventing it from crystallizing. These amorphous solid dispersions can be produced via HME or spray drying.
How HME Is Used: Because HME incorporates continuous mixing, it is inherently suitable for dispersing API into a polymer carrier. Feeding API and polymer into an extruder results in extrudate that contains stable, uniformly dispersed, soluble API. The material can then be further processed and combined with other excipients to create a finished dosage form. When compared to spray drying, HME benefits from being solvent-free, continuous, and easily scalable. Thermal degradation of an API is commonly cited as a reason to choose spray drying over HME, but formulation and process optimization can help overcome this challenge.
Controlled Drug Delivery
Why It Is Needed: Implantable drug-eluting systems (also known as drug-eluting devices) offer several unique advantages over conventional oral or parenteral drug delivery methods. For instance, they can provide localized, site-specific drug delivery, improving the effectiveness of treatment and minimizing side effects. These systems typically deliver API over an extended timeframe to provide continuous therapeutic effect with better patient compliance. This timeframe can vary widely depending on the product. Merck & Co.’s NuvaRing® vaginal ring delivers API for up to 3 weeks, while their Nexplanon® subcutaneous implant delivers API for up to 3 years (Figure 2).

How HME Is Used: Whether a drug-eluting system needs to deliver for weeks or years, many of these products rely on HME to disperse API in a polymer matrix that provides sustained, uniform drug release. HME is a well-developed processing technique that can leverage process analytical technology; therefore, it is possible to achieve customized and highly reproducible dispersions by this approach.
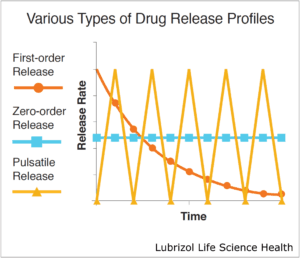
HME can produce drug-eluting systems with varied release profiles depending on the type of die that is employed (Figure 3). Simple extrusion of a solid profile produces matrix-type systems. These typically follow first-order release kinetics, often with an initial burst of drug. Reservoir systems, on the other hand, may utilize a tubing die that is then filled with drug. Some reservoir systems employ an advanced multi-layer extrusion technique wherein a drug-loaded core is simultaneously extruded within rate-controlling polymeric membranes. These systems achieve near zero-order release kinetics where the drug-release rate does not vary over time.
Other Uses
HME has also been explored for taste masking of bitter APIs. In these cases, the API is blended with a taste masking agent such as an ion-exchange resin. The interaction between the polymer and API shields the tongue from the bitter flavor of the API.
HME also provides a useful means for lifecycle management and/or intellectual property positioning. Innovators can extend the lifecycle of an API by using HME, such as by transitioning from a daily tablet to a sustained-release drug-eluting system. And intellectual property around a drug-eluting system can provide decades of value, as it has for Merck with their NuvaRing® product.
HME Equipment
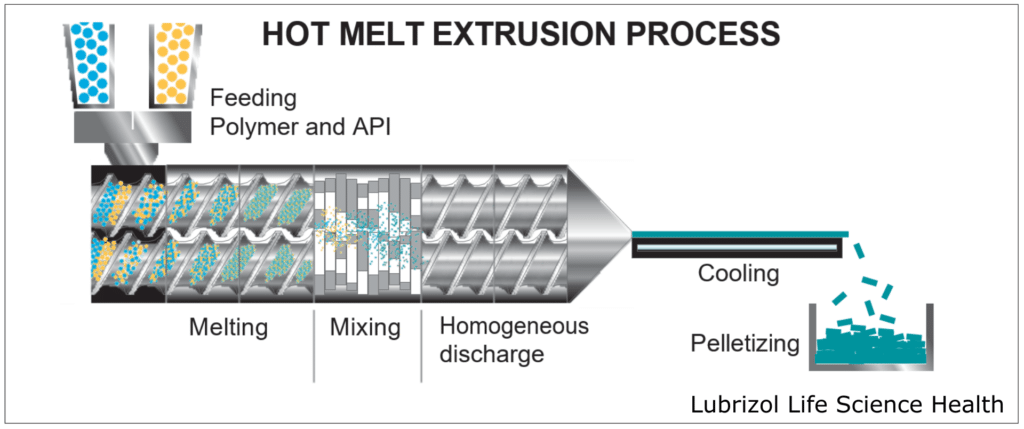
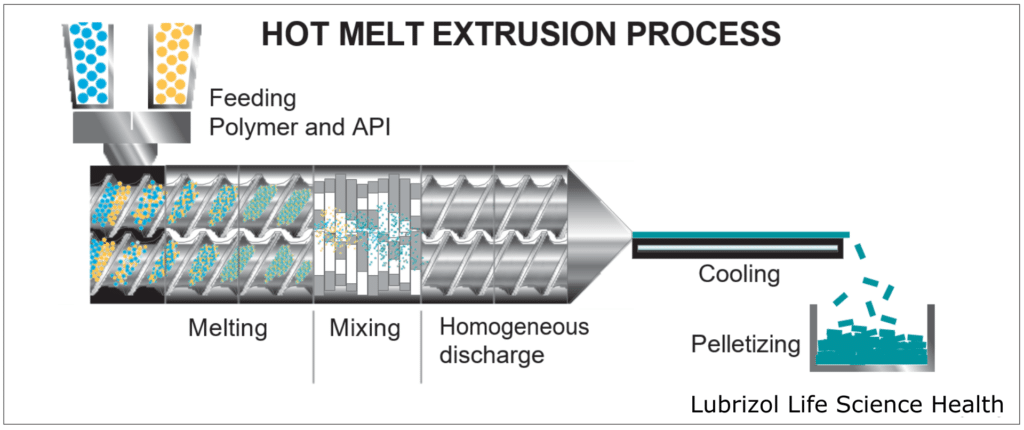
HME takes place within an extruder (Figure 4), which consists of a temperature-controlled barrel containing 1-2 rotating screws. As the screws rotate, they convey material through the barrel, melting and mixing the materials that are fed into the extruder. Extruders consist of four standard sections:
- Feeding: This is an opening where material enters the extruder. Either a hopper is attached to the opening for passive feeding, or the material(s) may be continuously pumped into the extruder by one or more external feeder(s).
- Conveying/Mixing: Consisting of the barrel and screw(s), this portion of the extruder introduces heat and shear force to the material(s), melting the feedstock and blending the materials into a homogeneous mixture as they move through the extruder.
- Extrusion: A die is attached to the end of the extruder, allowing for the materials to be extruded into a wide range of form factors, including rods, tubes, films, and sheets.
- Post-Processing: After extrusion, the material can be processed further to obtain a desired form. Some common processes include pelletization to create easily processed feedstock, milling to produce material for tableting, or injection molding to create a final dosage form.
The Twin Screw Extruder
While single screw extruders are useful for melting and conveying polymers, twin screw extruders are preferred for generating homogenous blends of materials such as polymer and API. Twin screws extruders are particularly useful when their screws intermesh and rotate in the same direction (co-rotate). Co-rotating, intermeshing screws ensure that materials are sufficiently mixed as they melt and are conveyed through the barrel.
HME Process Variables
A successful HME process involves co-interaction between many process variables. The graphic below (Figure 5) provides an overview of key variables, followed by a deep dive into each category.
Temperature
In a twin-screw extruder, melting of polymer is driven by shearing between the screws and frictional heating within the barrel. The barrel of the extruder is also temperature controlled to introduce additional heat for melting or to cool a process that is generating excess heat. Melt temperature (the temperature of the material in the extruder) is dependent on factors such as barrel temperature, screw speed, and feed rate. It is critical that process variables be optimized to achieve a melt temperature that lowers viscosity without thermally degrading the material. This is particularly important for thermally sensitive APIs.
Screw Parameters
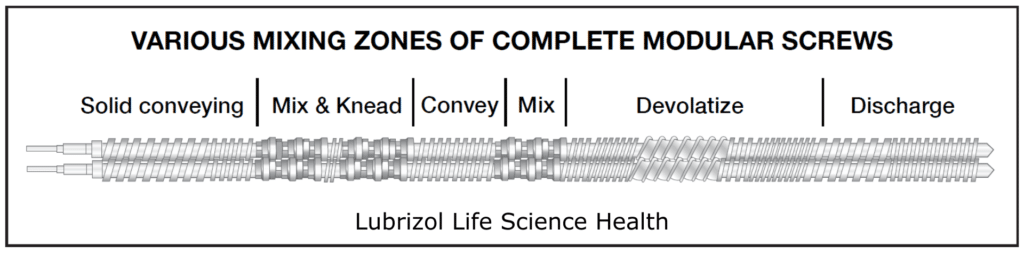
The screws within an extruder are designed to combine different types of mixing and conveying as material moves through the barrel. This is achieved with elements, which are modular pieces that comprise a complete screw (Figure 6). During product development, modular screws with multiple elements fitted on a common shaft allow the tailoring and optimization of the screw design for each product. Sections of the screw can be designed to perform particle-size reduction, mixing, and conveying functions. Single-piece production screws may be built to the same design as the development screws but are easier to clean for cGMP compliance.
Screw speed has an impact on transport, mixing, and energy introduced to the material in the extruder. During product development, varying screw speed allows one to measure its effect on mixing and final product characteristics. While higher screw speeds are desired to enhance mixing of material, increasing the speed also reduces residence time within the barrel. A shorter residence time can help to avoid thermal degradation of material. However, it can also lead to insufficient melting and an inhomogeneous dispersion, which is not acceptable for pharmaceutical products. Tracking residence time alongside final product characteristics allows developers to analyze the effect of screw speed and to find an optimal speed for their dispersions.
Exit Die Design
The use of different exit dies allows one to customize material for a specific dosage form. This adds to the versatility of HME, as dies can accommodate a range of form factors, including rods for subcutaneous implants and tubes for filled reservoir systems. An extrusion process may also include downstream processing such as cutting, spooling, or pelletization.
Materials for HME
Polymer Carriers
Typically, HME is used to combine an API with a carrier polymer. For most applications, the polymer should be thermoplastic, stable at the temperatures used in the process, and chemically compatible with the API during extrusion. For solid oral dosage forms, water soluble polymers are usually chosen from polymers already used in pharmaceutical products such as poly(ethylene glycol) and poly(vinylpyrrolidinone). For drug-eluting devices, the polymers are generally water-insoluble, and most products under development use either polyurethanes or ethylene vinyl acetate copolymers (EVAs). Both bioresorbable and non-bioresorbable polymers have been processed via HME.
APIs
While HME generates high temperatures to process materials, there are still a wide range of APIs that can be processed via this method, including many BCS class II and IV compounds, highly potent APIs, and controlled substances. The physicochemical properties of an API as well as the desired final dosage form are critical components of HME process design. This includes API solubility, melting temperature, physical state, lipophilicity, and thermal stability. Some APIs will exhibit thermal degradation at typical HME processing temperatures, so process optimization is required to protect the API. Interactions between an API and the carrier polymer can also change the properties of the mixture, which subsequently affects process parameters.
APIs that are processed in an amorphous state for solubility and bioavailability enhancement are particularly susceptible to stability issues, so the glass transition temperature of the mixture must be higher to prevent migration of the API over time. However, even APIs that are processed in a crystalline state for sustained release dosage forms can undergo polymorphic changes during processing, resulting in stability issues in the final dosage form. In these cases, adjusting the shear force on a material or altering the cooling rate as material exits the extruder can help control polymorphic behavior.
Processing Aids
Plasticizers help developers avoid thermal degradation by lowering the process temperature and reducing the residence time of material in the extruder. Common plasticizers include low molecular weight PEG, triacetin, and surfactants. Antioxidants such as vitamin E TPGS may also be introduced to prevent oxidative or free radical degradation and improve shelf life.
Conclusion
Hot melt extrusion is a versatile, proven technology for processing a wide range of dosage forms. It is a valuable formulation tool when it comes to enhancing solubility and bioavailability for BCS class II and IV APIs or designing drug-eluting systems for sustained release. Each component of the HME process is highly customizable, from the selection of a polymer carrier to the choice of an exit die for shaping material. However, a successful HME process relies heavily on having the proper equipment, personnel, and facilities. The level of investment and expertise needed to produce pharmaceutical-grade material cannot be taken for granted.
Particle Sciences has decades of experience with HME. Whether it’s a new chemical entity, a line extension (505(b)(2)), or a generic, we are equipped to handle the complete design, formulation, and production of our client’s drug-eluting systems. Our team is among the world leaders in formulating sustained-release dosage forms and has extensive experience using HME as part of a full suite of solubility and bioavailability enhancement tools.
Authors: