Lyophilization of Pharmaceuticals: An Overview
Table of Contents
Lyophilization transforms a drug product from a liquid to a stable solid by removing water or other solvents. Drug developers are increasingly interested in this technique as it can to extend the shelf life of both small and large molecule drugs.
Meeting the growing demand for lyophilization, also known as freeze drying, requires a high level of operational experience and practical expertise. Our Vice President of Operations, Karen Bossert looks at this technology and its uses.
What is Lyophilization?
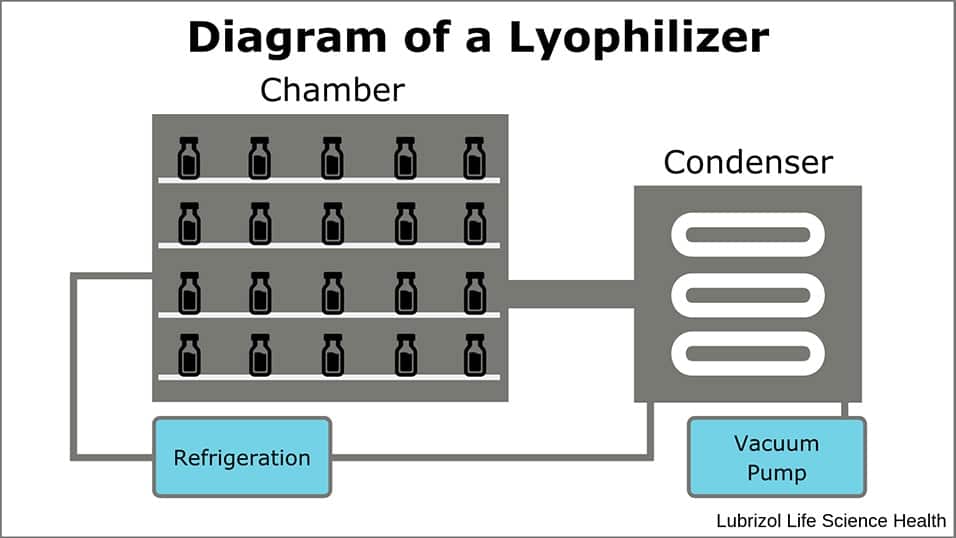
Lyophilization allows drug developers to stabilize formulations and therapeutic molecules through a commercially validated method. The process relies on the control of pressure and temperature in a lyophilizer (Figure 1) to remove liquid from formulations that consist of thermally sensitive or hydrolytically unstable active pharmaceutical ingredients (APIs) or formulation components. The resulting solid obtains greater stability than the aqueous solution and it can be stored for a longer duration at higher temperatures than its liquid precursor.
Large molecule developers find lyophilization particularly useful as lyophilized biologics do not require expensive, complex logistics such as rigorous cold-chain custody validation regimes and constant documentable refrigeration at the dispensary level.
Lyophilization consists of three interconnected stages:
- Freezing: During this step, the water or solvent in a product is gradually frozen by cooled shelves. This creates ice crystals that are separated from the drug product and more easily removed by sublimation.
- Primary Drying (Sublimation): During this step, pressure is manipulated to convert water directly from solid to gas via sublimation, and the resulting water vapor is collected on a condenser.
- Secondary Drying (Desorption): During this step, the shelf temperature in the lyophilizer is gradually raised under low pressure to drive off residual water or solvent. Careful consideration is given to ensure the temperature doesn’t exceed values at which product components are degraded or changed (this is especially important for thermally sensitive products like biologics).
At the end of the lyophilization process, a stable, solid material is obtained (typically described as a “cake”). Lyophilized products are often reconstituted at the bedside by healthcare professionals just prior to intravenous administration. They may also be incorporated into other dosage forms such as oral tablets.
Market Demand for Pharmaceutical Lyophilization
The prevalence of formulation stability challenges for complex APIs and biologics has resulted in more pharmaceutical and biotech manufacturers turning to lyophilization. The use of lyophilization for both pharmaceutical and biopharmaceutical manufacturing has grown around 13.5% per year over the last five years. And this pipeline of lyophilized products will only add to the established list of lyophilized drugs on the market today (Table 1).
Table 1: Global Sales of Top 10 Lyophilized Drug Products
| Product Name | API | Indication | Owner | Estimated 2018 Product Sales |
|---|---|---|---|---|
| Herceptin IV | Trastuzumab | Cancer | Genentech | $7.2B |
| Keytruda | Pembrolizumab | Cancer | Merck and Co. | $7.2B |
| Remicade | Infliximab | Rheumatoid Arthritis, Crohn’s Disease | Janssen Biotech | $6.4B |
| Botox | Daxibotulinumtoxin A | Various | Allergan | $3.6B |
| Carimune NF | Immunoglobulin | Immunodeficiency | CSL Behring | $3.3B |
| Xolair | Omalizumab | Asthma | Genentech | $3B |
| Orencia | Abatacept | Rheumatoid Arthritis | Bristol-Myers Squibb | $2.9B |
| Cosentyx | Secukinumab | Plaque Psoriasis | Novartis AG | $2.8B |
| Avonex | Interferon beta-1a | Relapsing MS | Biogen | $2.4B |
| Velcade | Bortezomib | Cancer | Takeda | $2.3B |
Many approved lyophilized drug products are biologics—proteins, peptides, nucleic acids, and other substances derived from or based on naturally-occurring compounds. It is estimated that over 60% of biologics on the market today would not be possible without lyophilization, and market demand for lyophilization technology will only increase as more biosimilars and novel biologics are developed.
Lyophilization as a Stabilization Tool
Lyophilization is particularly beneficial to parenteral drug developers, as a stable powder for injection can be easily packaged and transferred as a finished drug product. Lyophilization can also be employed to produce stable intermediates in drug product development and manufacturing. Hydrolytically unstable formulation components such as PLGA microparticles or fragile APIs may be lyophilized to create a longer shelf life and accommodate multi-step manufacturing processes. For example, APIs that undergo high energy media milling (AKA nanomilling) may be lyophilized prior to incorporation into an oral solid dosage form.
A Comprehensive Approach to Lyophilization
While lyophilization is considered a beneficial, commercially validated process, it also poses complex formulation and manufacturing challenges. The key challenges include:
- Specialized knowledge: Lyophilization cycles are not “one-size-fits-all,” and extensive cycle development is needed for each product. This may include a series of studies to understand the freezing and drying behavior of formulation components as well as investigations into how formulation strengths or containers affect the freeze-drying process. If lyophilized products contain other complex formulation components, such as nanoparticles, microparticles, or liposomes, additional formulation development and knowledge may also be needed.
- Additional contamination risk: Lyophilization leaves products exposed to the surrounding environment for extended periods of time, creating a high risk of contamination. As a result, sterile lyophilization must be performed in an ISO 5 (Class 100) environment, and many lyophilizers have Clean-In-Place (CIP) and Steam-In-Place (SIP) capabilities to support sterile and aseptic processes. Extensive validation and monitoring of cleaning procedures is required in any lyophilization operation.
- High capital investment: Large-scale lyophilization for sterile products requires multi-million dollar investments into equipment and facility maintenance (learn more about sterile manufacturing and aseptic processing here). As a result, both small and large pharmaceutical companies will often transfer their lyophilization processes to contract development and manufacturing organizations (CDMOs) for clinical and commercial manufacturing. CDMOs like Particle Sciences have the equipment and personnel in place to both develop and scale-up sterile lyophilization processes.
The Future of Lyophilization and Choosing a CDMO Partner
As the number of complex molecules in the drug development pipeline increases, more and more products stand to benefit from lyophilization. Any drug developer considering this manufacturing process must ensure that they have the combination of specialized knowledge, facilities, and equipment to achieve success. Any CDMO partner brought in to assist in a project must possess more than just the equipment – they need formulation and analytical expertise along with experience developing, scaling, and validating lyophilization cycles to ensure a project has a chance of success.
At Particle Sciences, we are leading the way in commercial aseptic manufacturing and sterile lyophilization of complex drug products, leveraging our decades of know-how as a leading product developer and clinical stage manufacturer. Our commercial facility features sterile lyophilization and is integrated into our existing development and clinical trial manufacturing site, offering customers a seamless flow from development through manufacturing.
Our facility contains ISO 5 Filling Suites for both clinical and commercial production. This includes our Grade A RABS aseptic filling suite, which can process vials, ophthalmic bottles, syringes, and cartridges. Our 120 ft2 lyophilizer builds upon our existing R&D and clinical lyophilization capability (2 R&D and 2 small-scale clinical lyophilizers) and is supported with both Clean-In-Place and Steam-In-Place capabilities for aseptic processing. Along with our sterile fill-finish and lyophilization capabilities, we can also perform particle size reduction and complex formulation activities under aseptic conditions. Finally, our analytical and quality control team works closely with our development and manufacturing staff to ensure your product is manufactured to the highest standards.
If you want to bring a complex, lyophilized drug product to market, look no further than the experts at Particle Sciences.
Authors: