
Nanomilling: A Key Option for Formulating Water-Insoluble APIs
Table of Contents
Key Points
- Nanomilling is the process by which the particle size of an API is reduced in a liquid vehicle (typically aqueous) via grinding using polymeric or ceramic media.
- Nanomilling is a proven, commercially validated process for formulating poorly water-soluble APIs. The key is the tremendous increase in surface area which directly correlates to an increase in dissolution rate.
- Media milling is a scalable, reproducible process for generating nanoparticulate suspensions.
- There are numerous processing and formulation considerations in nanomilling, each of which must be optimized on an API-by-API basis.
- Nanomilling requires unique expertise that is only developed through formulating a wide range of APIs
The Problem: Insoluble APIs
Poor water solubility is an increasingly common issue in pharmaceutical development. About 40% of marketed drugs and as many as 90% of active pharmaceutical ingredients (APIs) in the discovery pipeline are poorly water-soluble. When these APIs are introduced without modification or enhancement, they fail to dissolve and have limited bioavailability. As a result, there are multiple techniques available to increase the solubility of APIs and improve their delivery:
- Particle Size Reduction: nanomilling, micronization
- Amorphous Solid Dispersions: hot melt extrusion, spray drying
- Encapsulation Techniques: lipid- and polymer-based particles
- Other Solubilization Techniques: pH modification, salt forms, co-solvent systems, surfactants, complexation (cyclodextrin)
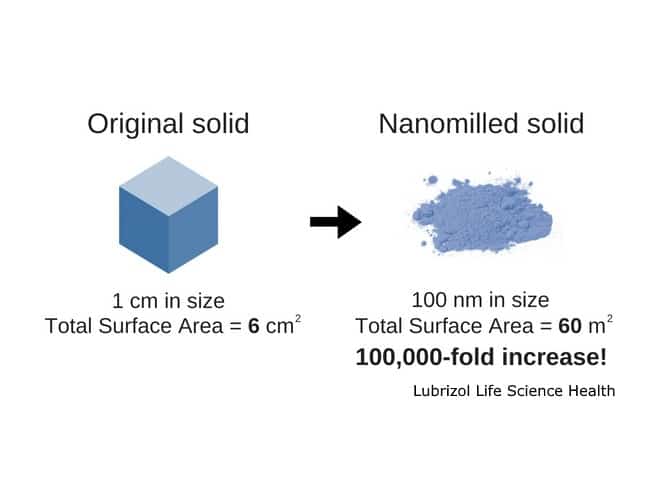
Nanomilling: A Proven Approach to Improving the Rate of Dissolution and Bioavailability
In our experience at Particle Sciences we’ve found nanomilling to be a particularly efficient, reproducible process that is very scalable. The basic principle behind nanomilling is increasing the surface area-to-volume ratio of an API by reducing the particle size below 1000 nm, typically in the 100s of nm range. This conversion of drug particles into nanocrystals allows for greater interaction with water, which increases dissolution rate. In general terms, smaller particles dissolve more quickly.
The technology behind nanomilling was developed in the 1980s and the first FDA-approved nanocrystal drug came to market in 2000. Since then, numerous BCS class II and IV APIs have benefitted from nanomilling and gained FDA approval (see table below). Nanomilling has been used to increase bioavailability and minimize fed/fasted variability in both liquid and solid dosage forms.
FDA Approved Nanomilled Drug Products (Updated September 2022)
| Brand | API | Dosage Form | Indication | Innovator |
| Rapamune® | Rapamycin, Sirolimus | Oral Solution Oral Tablet |
Immunosuppressant | Wyeth |
| Emend® | Aprepitant | Oral Suspension
Oral Capsule |
Anti-emetic | Merck & Co. |
| Tricor® | Fenofibrate | Oral Tablet | Hypercholesterolemia | Abbott Laboratories |
| Megace ES® | Megestrol | Oral Suspension | Anti-anorexic | Par Pharmaceuticals |
| Triglide® | Fenofibrate | Oral Tablet | Hypercholesterolemia | Sciele Pharma Inc. |
| Avinza®* | Morphine Sulphate | Oral Capsule | Pain | King Pharmaceuticals |
| Focalin XR® | Dexmethylphenidate HCl | Oral Capsule | Attention Deficit Hyperactivity Disorder (ADHD) | Novartis |
| Ritalin LA® | Methylphenidate HCl | Oral Capsule | CNS Stimulant | Novartis |
| Zanaflex® | Tizanidine HCl | Oral Capsule | Muscle Relaxant | Acorda |
| Invega Sustenna® Invega Trinza® Invega Hafyera® |
Paliperidone Palmitate | Intramuscular Injection | Schizophrenia,
Schizoaffective Disorder |
Janssen Pharma |
| Ryanodex® | Dantrolene Sodium | Intravenous Infusion | Malignant Hyperthermia | Eagle Pharmaceuticals |
| Aristada® | Aripiprazole Lauroxil | Intramuscular Injection | Schizophrenia | Alkermes |
| Yonza® | Abiraterone Acetate | Oral Tablet | Prostate Cancer | Sun Pharma |
| Anjeso® | Meloxicam | Intravenous Injection | Pain | Baudax Bio |
| VOCABRIA | Cabotegravir | Intramuscular Injection | HIV/AIDS | ViiV Healthcare |
| CABENUVA | Rilpivirine | Intramuscular Injection | HIV/AIDS | Janssen Pharma/ViiV Healthcare |
| Ztalmy® | Ganaxolone | Oral Suspension | Epilepsy | Marinus Pharmaceuticals |
* Discontinued
Advantages of Nanomilling
One of the greatest advantages of nanomilling when compared to other formulation approaches for poorly water-soluble APIs is its universality. While nanomilling is most useful for APIs with solubility below 200 µg/mL, it can be applied to just about any insoluble API in a relatively easy manner, making it an appealing first-line approach to solubilization. Other advantages include:
- NO harsh organic solvents or pH extremes: Most nanomilled suspensions are aqueous-based
- High API concentrations: 5-40+% API (w/w)
- Easy Scale-Up: Commercial nanomilling equipment utilizes a recirculation process that allows batch sizes to increase without changing process variables
- Reproducibility: Once a nanomilling process is optimized, there is minimal variation in particle size from batch-to-batch
“[Nanomilling] can be applied to just about any insoluble API in a relatively easy manner, making it an appealing first-line approach to solubilization.”
The Nanomilling Process
Nanomilling is a “top-down” approach to turning large, coarse particles into smaller, finer particles. It entails the application of mechanical energy to physically break down crystalline API structures. The main technique used for nanomilling API is high energy media milling. This technique relies on milling media—0.2-1 µm beads that are made from ceramics or highly crosslinked polystyrene. The beads shear and collide with the API particles during the milling process, reducing their particle size. During nanomilling, the API is suspended in a solution containing water and at least one stabilizer to prevent reaggregation of particles over time. The nanomilling process generates an intermediate consisting of nanoparticles suspended in an aqueous vehicle. This is often referred to as a nanoparticulate suspension (or nanosuspension). Nanosuspensions have been converted into a wide range of dosage forms, including oral liquids, capsules, tablets, films, injectables, aerosols, and more.
Media Milling: A Scalable, Reproducible Process
Media milling is the most commercially relevant process for creating nanosuspensions. In this process, the milling media is charged into a chamber containing an agitator. The API suspension is then pumped from a holding tank through the milling chamber, where the agitator rapidly rotates and creates turbulence in the media. As the suspension enters the milling chamber, the shear forces and impaction with milling media break down crystalline structures into smaller particles. The API suspension exits the milling chamber through a screen that keeps the suspension separate from the milling media. A coolant is pumped around the milling chamber to maintain a desired temperature. This is important for APIs that are thermally sensitive, as the milling process generates a significant amount of heat.
Media milling is relatively easy to scale up because process variables such as agitator speed and pump speed are independent of batch size. Larger batch sizes simply require longer milling times to ensure the suspension passes through the milling chamber enough to achieve the desired particle size. Media milling is also a highly reproducible process that results in little batch-to-batch variation once processing parameters are optimized.
Processing Considerations for Nanosuspensions

While the media milling process is well-documented, there are a number of processing parameters that must be optimized:
Agitator Speed
The speed of the agitator ultimately controls how much energy is being introduced to the suspension being milled. Higher agitator speeds (in the thousands of RPMs) result in a faster decrease in particle size because the number of collisions between particles increases. However, issues like temperature sensitivity, the level of milling impurities, and the speed at which an API mills affect the choice of agitator speed.
Media Load
Increasing the amount of media in the milling chamber can reduce milling time because it results in a higher number of collisions within the chamber. However, the media load must be tailored for each API based on the desired particle size reduction and the properties of the API.
Media Type
Multiple factors contribute to the selection of milling media type. For pharmaceutical applications, crosslinked polystyrene and ceramic media are the standard choices. Ceramic media is denser which means it can impart more energy to the system and reduce particle size for APIs with strong crystalline lattice structures. However, ceramic media also grinds the mill itself, leading to increased metal contamination in the nanosuspension. Because of this, polystyrene media is often used. Polystyrene media is less dense, which allows it to be run at higher speeds and for longer times without as much metal contamination.
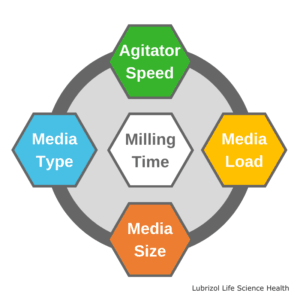
Media Size
The diameter of milling media beads in pharmaceutical processes typically ranges from 0.2-1 µm. Smaller media allows one to achieve lower particle sizes and reduces potential contamination compared to larger media. Studies have also shown that smaller media can reduce the time needed to reach a given particle size. The selection of media size is ultimately dependent on the desired final particle size of the nanosuspension.
Milling Time
The processing time needed for a nanosuspension is dependent on the desired particle size, the API being milled, the formulation, and a combination of all the above factors. In simpler cases where only a small reduction particle size is needed or an API mills quickly, processing time is relatively short. But in challenging cases where reduced milling speeds are required or an API has a high crystalline lattice energy, processing can take multiple days.
Formulation Considerations for Nanosuspensions
Nanosuspensions are generally comprised of water, stabilizer(s), and API. While these formulations avoid the use of organic solvents or other challenging excipients, nanosuspension formulation is not a straightforward task. Nanoparticles are inherently unstable due to high surface energy and tend to agglomerate over time, meaning the particle size will grow unless the suspension is stabilized. Nanosuspensions also exhibit Ostwald Ripening, a process by which differences in solubility cause smaller particles to preferentially dissolve and recrystallize onto larger particles. As a result, a great deal of work goes into the identification and selection of stabilizers. Below are some of the key considerations that go into stabilizer selection and nanosuspension formulation:
Physicochemical Properties of APIs
Surface energy, solubility, molecular weight, and crystal structure are just some of the API properties that affect particle size distribution and stability of nanosuspensions. Because each of these properties can impact stability, it is extremely difficult to standardize the selection of stabilizers based on the API alone.
Primary Stabilizer Properties
Polymeric stabilizers function by “coating” nanoparticles to improve their hydrophilicity and provide steric stabilization against particle aggregation. Primary stabilizer selection is a factor of viscosity and interaction with the API in question.
Secondary Stabilizer Addition
In some cases, a surfactant must be introduced to the formulation as a secondary stabilizer. Surfactants function by lowering surface tension and enabling wetting of particles, further improving nanoparticle solubility.
Stabilizer Concentration
Screening of stabilizers starts by testing standard concentrations of typical polymers. Based on the stability and particle size of samples, concentrations are adjusted and additional polymers or surfactants may be introduced. This iterative process of tweaking concentrations continues until a desired formulation is achieved.
Approval Status
The list of stabilizers available for formulation is largely dictated by the approval status of polymers. Based on the desired route of administration for an API, the list of generally recognized as safe (GRAS) stabilizers varies. For example, intravitreal injections have very few approved stabilizers. The oral route, on the other hand, has more options. If a stabilizer is introduced that has not been used before in a route of administration, additional work must be done to prove its safety and justify its use.
Concentration of API
The goal in any nanomilling process is to maximize API concentration, resulting in a more efficient process. Typical API concentrations range from 5-40+%, and the limiting factor is viscosity. API concentration can be adjusted along with stabilizer selection to maximize the amount of API while minimizing the viscosity of the nanosuspension (to allow for processing).
Analytical Characterization of Nanosuspensions
While every nanosuspension is unique, basic characterization is done via particle size distribution, zeta potential measurement, optical microscopy, and solid-state characterization.
Particle Size Distribution
Laser diffraction instruments measure particle size based on the angular intensity of scattered light in a sample. In general, a sample is dispersed in an aqueous medium and circulated through an optical cell through which two light sources (laser and visible lamp) are directed. Multiple detectors measure the intensity of light at various scattering angles and use the information to calculate a particle size distribution. This allows one to determine the mean, median, and range of particle sizes in a nanosuspension.
Zeta Potential
Measurement of zeta potential provides valuable information on the surface properties of nanoparticles, especially when ionic surfactants are used to stabilize a formulation. As a guideline, zeta potentials greater than +40 mV or less than -40 mV are generally sufficient to impart enough electrostatic repulsion to provide a stable nanosuspension.
Optical Microscopy
While nanoparticles cannot be seen with optical microscopy, it is a valuable characterization tool for nanosuspensions. Under optical microscopy, nanoparticles may appear as vibrating points within a suspension due to sufficient thermal energy to keep the particles discrete and in continual motion. This indicates that the particles are not aggregating to reach a lower energy state and the nanosuspension is physically stable.
Microscopy also allows one to see large aggregates or single API crystals that have grown through Ostwald ripening. It is important that these large particles are identified—especially if a nanosuspension is being developed for parenteral administration.
Solid State Characterization
The solid-state properties of the API should be assessed and monitored during the milling process. Some methods include x-ray powder diffraction, differential scanning calorimetry, FTIR spectroscopy, and Raman spectroscopy. In most cases, there is no change in morphology, but one can’t assume this will be the case and should generate this data during the development process.
Common Issues in Nanomilling
There are a range of issues that can arise during nanosuspension development. Overcoming these challenges requires extensive experience with troubleshooting the process and knowledge of how both processing and formulation considerations affect the final product.
- Lack of Stability: If the API can’t be stabilized, the suspension can become highly viscous, preventing milling.
- Overmilling: If an API is milled for too long, particle size can increase due to a number of factors that may include insufficient stabilizer concentration, aggregation, and/or Ostwald ripening.
- Changing Polymorphs: In some cases, high energy media milling can induce a polymorphic transformation of an API, so ways to lower the energy of the system must be explored. One may also want to start with the most stable crystal form.
- Combinations of Stabilizers: When individual stabilizers are insufficient, combinations are often successful in providing a stable formulation. Evaluating stabilizer interactions and ideal concentrations of combinations adds another level of complexity to the formulation process.
- Salt Sensitivity: Nanosuspensions that are stabilized by ionic surfactants are sensitive to changes in ionic strength, making it difficult to introduce salts such as tonicity modifiers. In these cases, carbohydrates or other non-ionic tonicity modifiers may be used.
“The greatest challenge of nanomilling is the fact that each processing and formulation variable must be optimized on an API-by-API basis. Nanomilling requires unique expertise that is only developed through formulating a wide range of APIs.”
Conclusion
Nanomilling is a proven, commercially validated process that we consider a first-line approach for the formulation of poorly water-soluble APIs. It is a versatile tool that can enable development of a wide range of dosage forms—both solid and liquid. As we explored in this post, there are numerous processing, formulation, and characterization considerations associated with nanomilling, resulting in a highly complex process. The greatest challenge of nanomilling is the fact that each processing and formulation variable must be optimized on an API-by-API basis. Nanomilling requires unique expertise that is only developed through formulating a wide range of APIs.
Particle Sciences is the leading CDMO for nanomilling. We have decades of experience developing nanosuspensions from scratch, both with commercial milling equipment and our own proprietary SteriMill™ technology. If you have an insoluble API, contact us today to learn how nanomilling can solve your formulation issues and let us take your product from concept to commercial.
Authors: