
How to Select the Right CDMO for Your Sterile Drug Product in 2023
Table of Contents
Scaling up production of your sterile drug product from clinical to commercial scale is a complicated and challenging process, with many factors to consider. This is particularly true when it comes to the scale-up of aseptic manufacturing processes, which are increasingly common in the pharmaceutical industry.
Finding the right manufacturing partner to bring your product from clinical to commercial production demands a careful balance of technical expertise and cultural fit. For example, many contract manufacturing organizations (CMOs) concentrate on high-volume products and may not offer the flexibility and partnership needed to scale up a new drug product. Other contract partners may offer great flexibility and collaborative personnel, but not have the right aseptic processing expertise or facilities for your product. Selecting a manufacturing organization requires a thorough evaluation of both science and talent.
The right partner is out there for commercial-scale aseptic manufacturing, but to find them, you need to know what to look for in a contract development and manufacturing organization (CDMO). In this blog, we will explore why it is so challenging to find a sterile manufacturing partner and highlight the key factors when selecting a CDMO partner for your project needs.
Where Sterile Manufacturing is Required
Sterility is required for several routes of administration. These include:
- Injection
- Ophthalmic
- Inhalation
- Otic
There has been steady growth in sterile manufacturing demand in recent years. Injectable drugs, such as therapies for cancer and biologics treatments accounted for 40% of new product approvals in 2020, and more than 50% of products in the innovation pipeline (see Table 1). Ocular drugs, meanwhile, have seen a 100% increase in clinical projects over the last five years. [1]
Table 1: Growth of Injectables in Clinical Pipeline
| Clinical-Stage Products | ||
| ROA | 2014/15 | 2020/21 |
| Injection | 46% | 51% |
| Oral | 40% | 38% |
| Topical | 5% | 4% |
| Ophthalmic | 2% | 2% |
| Inhalation | 3% | 2% |
| Nasal | 2% | 2% |
| Transdermal | 2% | 1% |
With this in mind, it is no surprise that it is challenging for drug product innovators to find sterile manufacturing partners, especially when moving from clinical to commercial scale. Not all CDMOs have the aseptic manufacturing capacity readily available to meet the needs of a new commercialization project or the flexibility to accommodate lower batch sizes for new products.
Key Considerations When Selecting a Sterile Manufacturing Partner
To find the right partner to ensure efficient and effective commercialization of your project, it’s important to take several factors into consideration. These include:
-
Quality
Quality is a key consideration when selecting any partner, but it is absolutely critical when it comes to sterile manufacturing and demonstrating compliance with stringent global regulatory requirements. A CDMO should be fully aware and expert in the regulations in place in all the key markets where you intend to sell you product to ensure that it is fully compliant.
A good CDMO should also be able to demonstrate to you that it has a strategy for maintaining a high standard of quality over time. This means the CDMO should have an established quality manual, employee training program, and continuous improvement processes. An inadequately trained manufacturing team or an inability to resolve issues such as deviations can be costly or even devastating to a drug product.
Your CDMO should also be able to provide evidence that they have protocols to ensure and demonstrate data integrity, so you can be confident in the documentation they provide you for regulatory bodies.
Finally, a culture of accountability is an important feature in a manufacturing partner. People in accountable organizations demonstrate high levels of ownership and think/act in a manner that drives organizational results. A culture of accountability has a powerful impact on project success and adds real value to the partnership between CDMO and client.
-
Right-Sized Capacity and Security of Supply
It is crucial to ensure you select the right-sized CDMO for the needs of your aseptic project. If the CDMO is too small, they may not have the dedicated manufacturing capacity or the secure supply chain required to scale and deliver the volumes you need on schedule. Too large, and they may struggle to give your project the attention it deserves, potentially resulting in delays.
Selecting the right-sized CDMO requires you to consider the following:
- Does the CDMO manufacture the required batch sizes?
- Does the CDMO have the available, dedicated sterile capacity to deliver on time?
- Does the CDMO offer a personalized, tailored approach?
- Does the CDMO offer security of supply?
- Is the CDMO invested in your long-term success?
An ideal CDMO not only has the available capacity and the supply chain security to meet your needs, but they offer a team that cares about your product as much as you do, regardless of your batch sizes or the size of your company. For example, Particle Sciences has no minimum batch sizes and treats every project with the same personalized, responsive touch that we’ve developed over the past 20+ years. Learn more here.
-
Flexibility and Transparency
Every manufacturing project has challenges, whether they stem from the sourcing of raw materials, the scale-up of manufacturing steps, or the complexities of qualifying equipment. Some CDMOs are rigid and inflexible when it comes to their infrastructure, capacity, and internal processes, and don’t always communicate their limitations or potential challenges.
Nevertheless, successful commercialization requires both transparency and flexibility from a partner. An ideal CDMO will be upfront with you about these challenges and will work with you to find creative ways to circumvent them and ensure the smooth and timely delivery of the project.
Good manufacturing organizations proactively plan and prepare for long-term projects (see “Risk Mitigation” below), but not every project is going to be a perfect fit from start to finish. Alignment on the time and resources needed to meet both current and future needs is critical, and you should ensure your CDMO shares the goal of supporting your project both today and into the future.
Your CDMO should also be realistic in terms of the timelines of a project, especially when it comes to aseptic processing. If a project timeline sounds too good to be true, then it probably is!
Figure 1: Key Considerations When Selecting a CDMO
-
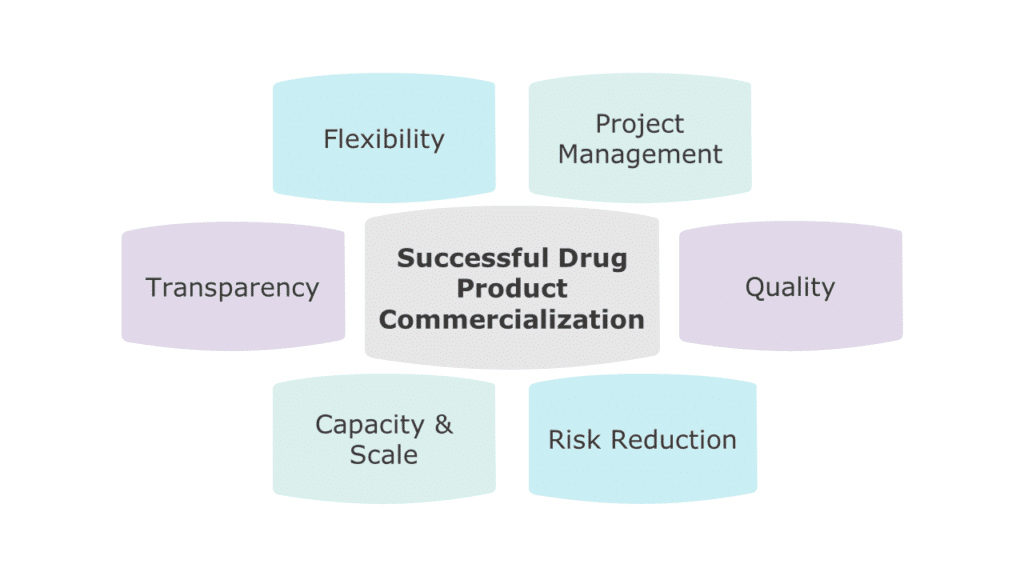 Risk Mitigation
Risk Mitigation
To ensure the partner can mitigate risk for sterile projects, it is crucial to evaluate a CDMO’s media fill/aseptic process simulation (APS) strategy. CDMOs that offer a “bracketed” range of compounding processes, container closure systems, and fill volumes can “plug-in” many aseptic manufacturing projects quickly and efficiently, saving customers time and money. A willingness to utilize single-use systems in addition to dedicated equipment can also increase efficiency and reduce manufacturing timelines.
Ask if the facility is FDA-inspected and determine if the CDMO has successfully completed any pre-approval inspections (PAIs). A successful PAI indicates that the CDMO is capable of manufacturing a drug product with accurate and complete data. Review relevant data from past PAIs and determine if the CDMO’s PAI history has weight relative to your project.
In addition to reviewing FDA inspection history, understanding a CDMO’s history of tech transfers and work on similar projects is vital. This will indicate whether the organization can troubleshoot challenges and has the experience to deliver your project effectively.
Particle Sciences has completed a successful PAI and has a long history of tech transfers, putting us in an ideal position to manufacture commercial drug products both today and into the future.
-
Cultural Fit
Finally, relationships matter when selecting a sterile manufacturing partner. In addition to meeting all the requirements above, it is important to select a CDMO that you trust will align with your goals and act as a true extension of your team.
This starts with your interactions with business development personnel, who should be responsive and open when discussing your project. Ideally, your request will be handled by a dedicated representative from the CDMO who can build an understanding of your needs and translate them into a realistic project proposal.
Another major part of building trust is understanding the CDMO’s approach to project management. It is important to understand how a CDMO will communicate throughout a project. What is the cadence of team meetings? Who will be my point of contact throughout a project? Will my project manager be able to accurately convey my technical needs to the project team?
Lastly, an ideal CDMO should take a vested interest in your project’s success. Team members should take personal responsibility for meeting project milestones and provide timely, accurate responses to questions/concerns throughout a project.
At Particle Sciences, we assign a team of dedicated experts from project management, manufacturing, formulation, and analytical services for each project, augmenting with tech transfer and quality resources as necessary. Our project managers also have technical backgrounds and understand the science behind processes, which ensures smooth communications to the larger team. Particle Sciences prides itself on acting as an extension of our clients’ teams by combining talent, technology, flexibility, and communication to build long-term relationships.
Finding the Right Sterile Manufacturing Partner
Developing, scaling, and taking a sterile drug product to market is a challenging journey. A great deal of time and money is invested in every project, so it is crucial to identify a manufacturing partner that increases your chances of success. Balancing the considerations above will go a long way in helping you select a partner who you can trust with your next drug product.
At Particle Sciences, we provide a personalized approach to aseptic/sterile manufacturing:
- Our FDA-inspected facility offers sterile filling with no minimum batch sizes, ensuring that your project will get the attention it needs.
- Our team values honest, two-way communication and takes pride in the successes of each project—big or small.
- Our culture of accountability and problem-solving ensures we are quick to troubleshoot issues and keep your project on track.
Authors: Robert Lee and Nick DiFranco