Services
Learn more about what Agno Pharmaceuticals can do to help you accomplish your goals.
Feasibility Screens
Highly Potent APIs and DEA Controlled Substances
Cryomilling
Wide Array of Form Factors
Experienced Analytical Team to Fully Characterize these Dosage Forms
From Concept Through Commercial Manufacturing

Our Collaboration With Partners
Agno Pharma collaborates closely with partners to address the challenging formulation and development needs of drug-device combination products.
Our comprehensive services encompass design, formulation, and manufacturing stages essential for achieving therapeutic objectives. Whether aiming for sustained drug release, enhanced targeted delivery, reduced dosage frequency, or improved patient adherence, our teams offer specialized expertise and capabilities to support every phase of development. We excel in advanced technologies such as Hot Melt Extrusion (HME) for efficient formulation of drug-device combinations, Cryomilling for precise particle size reduction optimizing API dissolution rates, and injection molding ensuring scalable production of precise drug-device components.
At Agno Pharma, our encapsulation techniques including microsphere and nanoparticle technologies provide versatile solutions for encapsulating APIs, enhancing stability, and enabling targeted delivery. Agno Pharma combines technical proficiency with a commitment to innovation, delivering tailored solutions that meet exacting standards of efficacy, safety, and patient satisfaction.
Additional Services
Agno Pharma excels in leveraging Hot Melt Extrusion (HME) as a robust technology to address complex formulation challenges, particularly for enhancing solubility and achieving controlled drug release profiles. HME involves the application of heat and pressure to melt polymers and APIs, facilitating their uniform dispersion and subsequent formation into various dosage forms. This continuous and reproducible process is pivotal in the pharmaceutical industry for formulating poorly water-soluble APIs, including BCS class II and IV compounds, as well as highly potent and controlled substances.
Key Capabilities of the Bethlehem Facility’s HME Expertise:
- Enhanced Solubility and Bioavailability: Agno Pharma employs HME to convert APIs into amorphous solid dispersions, thereby enhancing their solubility and bioavailability by preventing crystallization and improving dissolution rates.
- Controlled Release Systems: Utilizing HME, Agno Pharma designs and manufactures systems that offer sustained and targeted drug delivery. This includes developing matrix-type and reservoir-type systems with precise release kinetics suitable for various therapeutic needs.
- Versatility in Dosage Forms: With HME, Agno Pharma produces a wide array of form factors such as rods for subcutaneous implants, tubes for reservoir systems, and films for transdermal patches, catering to diverse pharmaceutical applications.
- Process Optimization: Agno Pharma applies rigorous process optimization strategies to mitigate challenges such as thermal degradation of APIs and polymer interactions, ensuring the stability and efficacy of the final dosage forms.

Our commitment to advanced technologies like HME is underscored by our comprehensive capabilities in design, formulation, and manufacturing of pharmaceutical-grade products. Whether for new chemical entities, lifecycle management strategies, or generic formulations, our specialized expertise in HME enables us to deliver innovative solutions that meet stringent regulatory standards and exceed client expectations. Contact us to explore how Agno Pharma can collaborate with you to optimize your drug formulation challenges using state-of-the-art HME capabilities.
To learn more about HME, please visit the link
Cryomilling, also known as cryogenic milling or cryogrinding, is a specialized mechanical process used to reduce the particle size of polymers and other materials by cooling them to cryogenic temperatures, typically using liquid nitrogen. This approach offers several distinct advantages, particularly in the pharmaceutical industry where precision and uniformity are critical for drug delivery device manufacturing. By achieving finer particle sizes through Cryomilling, Agno Pharma enhances the flowability and processability of polymers, facilitating more consistent and effective dispersion of active pharmaceutical ingredients (APIs) within polymeric matrices. This process is essential for developing implantable drug delivery systems, intravaginal rings, and other controlled release devices where uniform distribution of APIs ensures optimal therapeutic outcomes.
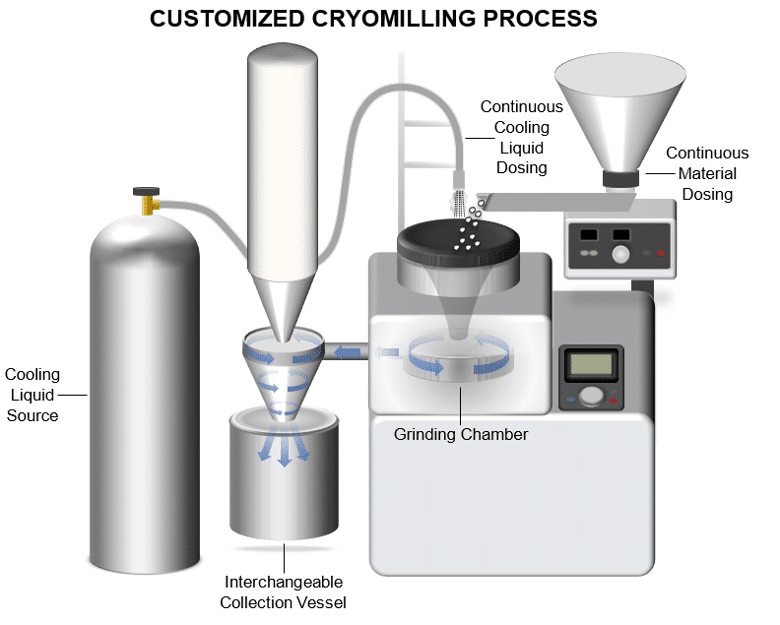
Key Benefits of Cryomilling:
- Enhanced Homogeneity: Cryomilling enables the creation of uniformly blended polymer-drug mixtures, crucial for products containing highly potent APIs that require precise dosing. It ensures even dispersion of drugs throughout the polymer matrix, enhancing both safety and efficacy.
- Improved Safety and Compliance: By operating under cryogenic conditions, Cryomilling minimizes the risk of thermal degradation or polymer melting, preserving the integrity of both polymers and APIs. This temperature control is essential for meeting stringent regulatory standards and ensuring pharmaceutical-grade quality.
- Scalability and Efficiency: Our team has developed advanced Cryomilling processes that support scalable production from small batches for clinical trials to larger volumes for commercial manufacturing. This scalability ensures consistent product quality and accelerates the development timeline for pharmaceutical formulations.
Agno Pharma leverages its expertise in Cryomilling alongside comprehensive drug development capabilities, including formulation, extrusion, and clinical production. This integrated approach enables us to deliver innovative drug delivery solutions tailored to meet the unique requirements of our clients, from concept through to commercialization. Whether for implantable devices or 3D-printed pharmaceuticals, Agno Pharma stands ready to optimize drug release profiles and device formats through cutting-edge Cryomilling technologies.
To learn more about our Cryomilling capabilities, please visit the link.
At Agno Pharma, injection molding capabilities play a crucial role in our comprehensive suite of drug delivery technologies. We leverage injection molding to precisely manufacture components for various drug delivery systems, including implants and injector devices. This technology enables us to produce complex shapes and structures with high precision and repeatability, meeting the stringent requirements of pharmaceutical applications. Whether it’s creating implantable dosage forms or specialized injector devices, our injection molding capabilities ensure that we can tailor solutions to meet specific therapeutic needs and performance criteria.
Our state-of-the-art Bethlehem facility is equipped with advanced machinery and operates under strict quality control measures to ensure compliance with regulatory standards. This allows us to consistently deliver high-quality components that are integral to the functionality and performance of our clients’ drug products. At Agno Pharma, injection molding is not just a manufacturing process but a critical enabler of innovative drug delivery solutions, supporting our commitment to advancing pharmaceutical technologies that improve patient outcomes and treatment efficacy.

To learn more about our capabilities, please follow this Link
Agno Pharma excels in encapsulation capabilities, making us a trusted partner for a wide range of pharmaceutical and biopharmaceutical applications. Here’s why our encapsulation expertise stands out:
- Advanced Technologies: We leverage state-of-the-art technologies such as nanomilling, microsphere production, and microfluidization (i.e., high pressure homogenization) to encapsulate active pharmaceutical ingredients (APIs) efficiently. These technologies ensure precise control over particle size, distribution, and encapsulation efficiency, critical for achieving desired therapeutic outcomes.
- Customized Formulation Solutions: Our approach to encapsulation is highly tailored to meet specific client needs. Whether it’s enhancing API solubility, improving bioavailability, or achieving controlled release, we design formulations that optimize drug delivery and efficacy.
- Broad Application Scope: Agno Pharma caters to diverse encapsulation needs across various dosage forms, including oral, parenteral, and topical applications. From developing sustained-release formulations to complex biopharmaceuticals, we have the expertise to handle a wide spectrum of projects.
- Quality and Compliance: When requested, we adhere strictly to cGMP guidelines throughout our encapsulation processes, ensuring the highest standards of quality, safety, and regulatory compliance. Our facilities are equipped with ISO 5 aseptic filling suites and state-of-the-art analytical capabilities to support robust formulation development and manufacturing.
- Experience and Expertise: With decades of experience in pharmaceutical development and manufacturing, Agno Pharma has a proven track record of successfully encapsulating challenging APIs, including small molecules, peptides, proteins, and nucleotides. Our team of formulation scientists and engineers brings deep expertise and innovation to every project.
- End-to-End Solutions: From early-stage feasibility studies to commercial-scale production, our team offers comprehensive encapsulation services under one roof. We support clients from concept through commercialization, providing seamless integration and accelerated time-to-market for pharmaceutical products.
Partnering with Agno Pharma for encapsulation ensures access to cutting-edge technologies, customized formulation solutions, and a commitment to excellence in pharmaceutical development and manufacturing.
