
Biopharmaceutical Classification System
Table of Contents
The Biopharmaceutical Classification System (BCS) is an experimental model that measures permeability and solubility under prescribed conditions. The original purpose of the system was to aid in the regulation of post-approval changes and generics, providing approvals based solely on in vitro data when appropriate. Importantly, since the majority of drugs are orally dosed, the system was designed around oral drug delivery. Waivers (i.e. permission to skip in vivo bioequivalence studies) are reserved for drug products that meet certain requirements around solubility and permeability and also rapidly dissolve in the human body.
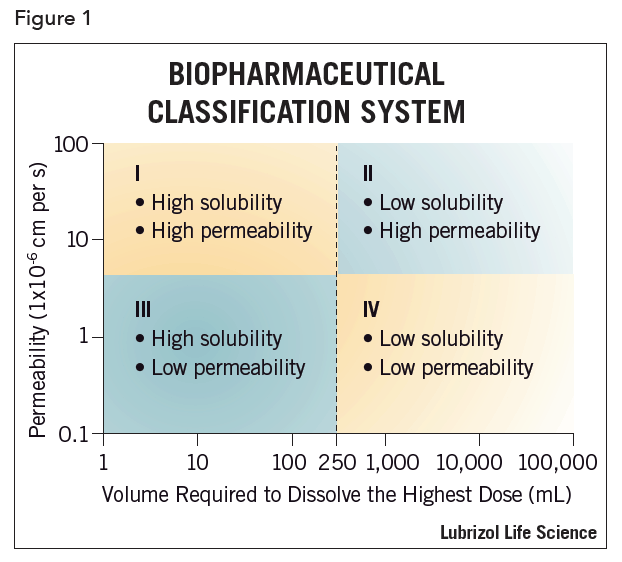
More and more however, the industry is using the BCS as a tool in drug product development. This system can be used to flag drugs that should not be tested clinically unless appropriate formulation strategies are employed (see Figure 1). For example, a BCS Class II compound (permeable but relatively insoluble) would likely not be a good clinical candidate without the use of enhanced formulation techniques aimed at increasing solubility or rate of dissolution. Various schemes exist that attempt to funnel a given active pharmaceutical ingredient (API) towards a particular drug delivery technique based on the BCS category. Still, most approaches remain fragmented in their methodology, ignoring commercially and biologically important factors. The BCS can, however, when integrated with other information, serve as an effective tool for efficient drug development. One school of thought is that first in human (FIH) drug dosage forms should be designed to maximize bioavailability. The FIH dosage form should be a logical step towards commercialization and not simply a stop gap to facilitate data acquisition.
 For BCS Class I molecules, FIH formulations are straightforward and may consist of essentially the neat API. For other compounds, effective dosage forms present greater challenges. Although designed originally to classify APIs by their oral bioavailability, when properly augmented the BCS can be used as a key component of an algorithm to guide drug delivery system design for any route of administration.
For BCS Class I molecules, FIH formulations are straightforward and may consist of essentially the neat API. For other compounds, effective dosage forms present greater challenges. Although designed originally to classify APIs by their oral bioavailability, when properly augmented the BCS can be used as a key component of an algorithm to guide drug delivery system design for any route of administration.
The BCS
The BCS places a given API in one of four categories depending on its oral dosing solubility and permeability (see Figure 1). A drug substance is considered “highly soluble” when the highest clinical dose strength is soluble in 250 mL or less of aqueous media over a pH range of 1–7.5 at 37°C. A drug substance is considered to be “highly permeable” when the extent of the absorption (parent drug plus metabolites) in humans is determined to be ≥90% of an administered dose, based on a mass balance determination or in comparison to an intravenous reference dose2.
Permeability can be determined a number of ways but is most often done using Caco-2 cell lines, an assay that lends itself to high throughput automation. In this system a monolayer of cells is grown and drug permeation from the drug donor (apical side) to the acceptor (basolateral side) compartments is assessed, usually by direct UV or LC-MS assay. Potential issues with Caco-2 based systems range from variation (from in vivo) in transport mechanisms to drug interactions with the apparatus itself. Commercial companies focused on this assay have developed multiple approaches to alleviate these issues, but a discussion on the subject is beyond the scope of this technical brief. As a drug candidate moves up the development ladder, developers will often confirm and refine their BCS assessments with increasingly complex in vivo models.
An important factor to remember with the BCS is that it accounts for potency in that solubility and permeability are relative to clinical dose. Again, oral dosing is assumed in the testing design. So, for example, a compound that has poor absolute solubility might paradoxically be classified as “highly soluble” if it were a highly potent compound and the whole clinical dose was soluble in 250 mL.
BCS and Dosage Form Trends
It is commonly recognized that most new drugs present formulation challenges. Older drugs as compared to newer ones generally have higher solubilities. One reference noted that BCS Class II compounds, as a percentage of the total number of compounds in development, has increased from 30% to 60%. BCS Class I compounds have fallen correspondingly from 40% to 20% over that same period3. In practice, low solubility is the most common theme encountered. It should be noted that not every drug is classified the same by each investigator. The variability can be due to a number of things including the way permeability is measured. As above, in vivo permeability is impacted by, among other things, drug transporters. Both uptake and efflux transporters exist and can contribute to the differences seen by the various techniques.
For the majority of APIs, a solid oral dosage form (SOD) is the preferred option. Sometimes the physicochemical and physiologic mechanisms do not allow this and alternatives such as oral suspensions or solutions are pursued. Other times, the target and additional factors dictate that a non-oral dosage form is most sensible. Examples include localized delivery of female hormones, nasal allergy preparations, ocular therapeutics, and combination products aimed at prolonged drug release. In all these cases, even though not orally dosed, the concepts inherent in the BCS can be important tools in dosage form design.
Formulation Approach
Having a pre-defined system in which one can make decisions based on data is necessary for efficient drug development. Inputs into such a system include, in addition to BCS class, a detailed solubility profile, polymorph status, desired dosage form, target dose and dosing regimen, drug stability, excipient compatibility, and knowledge of transporter and metabolic pathways. Non-technical factors that, as a practical matter, need to be considered are such things as cost, intellectual property and distribution chain limitations. Integration of these into a methodical systematic approach will maximize the chances of a successful outcome. As R&D dollars become ever more scarce, it becomes increasingly evident that early consideration of as many factors as possible can be critical, regardless of the route of administration. In practice, this leads to the strategy of getting to FIH as quickly as possible with a formulation strategy that accounts for both physicochemical properties and physiologic influences.
A complete set of algorithms covering the four classes and all possible dosage forms is well beyond the scope of this tech brief. However, a few fundamental principles can be covered. First, it is critical to characterize your compound. Understanding the basic behavior of a given compound in various solvents and across a range of pHs is fundamental to designing a dosage form. For instance, a compound soluble only at lower pHs will require a different formulation than one freely soluble at an intermediate pH. Likewise, a soluble yet impermeable compound will require yet another strategy. Very importantly, this is true whether one is administering the drug, for example, IV or orally. The formulation implications differ with route of administration but the necessity of accounting for these properties is universal. It is important that the drug developer be equipped with a range of technologies that can address the various patterns that emerge. Nothing wastes more time and money than trying to fit a drug to a specific preordained delivery technology.
Armed with the proper set of tools, one can rapidly narrow down the potential approaches. For the most part, all drug delivery strategies are trying to control drug exposure. Often, one is trying to maximize it over time i.e. area under the curve (AUC) and/or concentration i.e. Cmax, but the aim of extended release and/or site-specific delivery is also common. In addition to the delivery goals, other functions are often required such as API stabilization or taste masking. In short, no one formulation approach will ever satisfy all or even a substantial portion of drug delivery demands.
For oral drug delivery, a simplified summary of approaches based on properties might look like Table 1. Each approach must then be tailored to meet the other demands of that particular API and desired product profile.
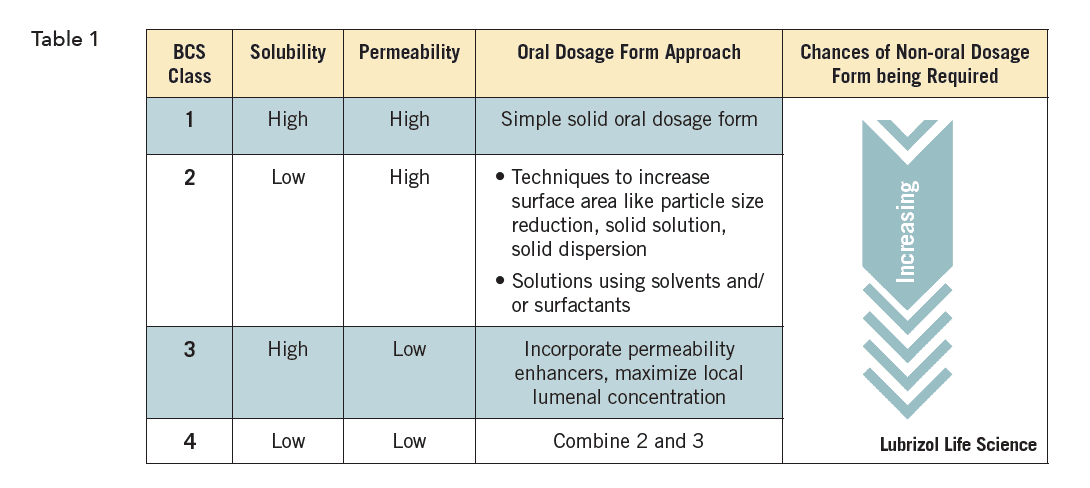
Similar charts exist for virtually all routes of administration. Each route of administration will of course have different options, but they are regardless all ruled by the interplay of the drug’s physicochemical properties and the local and systemic physiology they encounter.
Independent of the final dosage form, ideal drug development involves an iterative process of setting goals, performing formulation work, and developmental stage appropriate testing. Early on, after physicochemical evaluations are complete, BCS and polymorph screening might be performed. After thorough pre-formulation, including solubility and stability testing, early formulations might again be screened for their impact on dissolution or bioavailability. This approach is repeated such that at each inflection point, data is gathered to support the development plan. In this way, FIH is achieved most efficiently and in a way that insures clinically relevant data is obtained.
References
- Chi-Yuan Wu and Leslie Z. Benet, Predicting Drug Disposition via Application of BCS: Transport/Absorption/Elimination Interplay and Development of a Biopharmaceutics Drug Disposition Classification System, Pharmaceutical Research, January 2005, 22(1), 11-23.
- M. Sherry Ku, Use of the Biopharmaceutical Classification System in Early Drug Development, AAPS J., March 2008, 10(1), 208–212.