
Dissolving Films
Table of Contents
The pharmaceutical industry is no stranger to the use of thin polymer films for drug delivery, as many prescription and over-the-counter treatments come in the form of transdermal patches. Because the oral delivery route remains the preferred route for administering therapeutic agents, the idea of the orally-dissolving film as a drug delivery vehicle has attracted considerable attention in recent years.
Having originally been developed as a novelty confection, the orally-dissolving film has seen application in personal care products (Listerine PocketPaks®) and in some over-the-counter medications (TheraFlu Thin Strips®, Sudafed PE Quick Dissolving Strips®, Gas-X Strips®). Dissolving film products have also been developed for delivery routes other than oral, such as vaginal (VCF Vaginal Contraceptive Film®).
General Attributes
The dissolving film delivery vehicle is essentially just a thin, flexible sheet of polymer in which an active pharmaceutical ingredient (API) has been incorporated. Depending on the nature and desired dosage that the film is to deliver, the API may be either dissolved or suspended as crystals or amorphous particles in the polymer matrix of the film.
Size and thickness of a dissolving film product is largely dependent on the dosage of API that it intended to deliver, but the physical attributes are also influenced by the disintegration characteristics that the film is intended to have; a higher surface-to-mass ratio for a strip of film affords a more rapid disintegration time than a lower one. In general, orally-dissolving films tend to be a few square centimeters in surface area and are typically 50 to 150 μm thick. These dosage forms are usually intended to dissolve as quickly as possible, on the order of a few seconds. Films that are designed for vaginal delivery are often larger and thicker because disintegration time is not as critical as in the case of oral delivery films and increased residency in the vaginal cavity is often more desirable. Film texture is primarily a matter of consumer preference, and the physical feel and pliability of the film can have an influence on patient compliance. In the case of vaginal films, texture is a significant concern because it directly affects the ease of application.
Advantages of Dissolving Films
Dissolving films, as a drug dosage form, encompass many of the benefits of both liquid and solid dosage forms. Like a tablet, a film’s physical structure and its low moisture content makes it an inhospitable environment for microbial growth, which minimizes the need to incorporate preservatives and affords greater stability and shelf life. Unlike traditional solid dosage forms, however, thin, flexible strips of polymer are not friable, allowing them to resist the kinds of physical degradation that would damage normal tablets and capsules. Greater stability is also afforded by the packaging of the films; film strips can be individually wrapped in flat, sealed packages that are practically devoid of air. This means that they are exposed to atmospheric moisture and oxygen only at the time of administration.
Dissolving strips are also discrete and easily portable dosage forms. Single doses of medicine can be carried individually, without the need for a secondary container to protect or contain them, and without the need for an appliance with which to administer them, such as with vaginal gel applicators.
In contrast with traditional solid dosage forms, like tablets and capsules, a strip that is administered, either orally or vaginally, dissolves rapidly in the ambient mucosal moisture and the resultant hydrated material functions with the same rapid-release characteristics as would a typical liquid suspension or hydrogel formulation.
Films that are intended for vaginal administration can be applied manually, without the mess or the inconvenience of a gel or cream applicator. Upon contact with vaginal fluid, the film-forming ingredients hydrate to form an ad hoc hydrogel that functions as would a normal vaginal gel product. The use of film-forming ingredients that also have mucoadhesive properties helps the formulation to remain at the administration site after hydration.
One of the primary benefits of an orally-dissolving film product is the ease of administration; a thin strip of film can be taken by mouth, easily and discretely. No water is needed to swallow a dissolving film, making it a useful delivery form for medications intended for pediatric, geriatric, or dysphagic patients who may have difficulty swallowing a pill. Additionally, with the use of taste-masking compounds, flavor-receptor blocking, and microencapsulation technology, where the API is enveloped in a barrier material on a molecular level, even bitter, unpalatable APIs can be used successfully in an oral film formulation.
A dissolving film can be manufactured with a higher proportion of APIs than would be possible in a traditional formulation without compromising its physical integrity. In fact, high levels of suspended solids can help stabilize the film matrix in much the same way that suspended solids can help to stabilize a traditional emulsion. Given the relatively low mass of a strip of polymer film, however, even a high level of loading may not provide a sufficient dose for certain APIs.
Film Manufacture
Dissolving films are comprised primarily of water-soluble polymers, such as polyethylene oxide, hydroxypropyl methylcellulose, methylcellulose, carboxymethyl-cellulose, polyvinyl pyrrolidone, gelatin, pectin, and pullulan. They comprise the physical structure of the films, affording their integrity. Polymers are selected not only for the physical characteristics that they impart to the films but also for the rate at which they dissolve. The dissolution rate of a dissolving film is inversely related to the molecular weight of the film polymers and will, in turn, dictate the rate at which the medicine is delivered. Fine-tuning a dissolution rate can be as simple a process as varying the concentration of two different molecular weight polymers.
Plasticizers, such as glycerin, propylene glycol, and poly(ethylene glycol), can be added to the formulation to alter the mechanical properties of the final film. By lowering the glass transition temperature of the film polymers, the resultant films not only have more pleasant textural characteristics but also are stronger and more flexible. Other ingredients, such as taste-masking compounds and preservatives, are added as needed, but often in lower quantities than would be needed for a liquid dosage form because the dissolved film, hydrated with trace amounts of mucosal moisture, is highly concentrated.
Two methods predominate in the manufacture of thin films: solvent-casting (Figure 1) and hot-melt extrusion (Figure 2). Both have benefits and drawbacks, contingent on the nature of the APIs used and the desired characteristics of the final product.
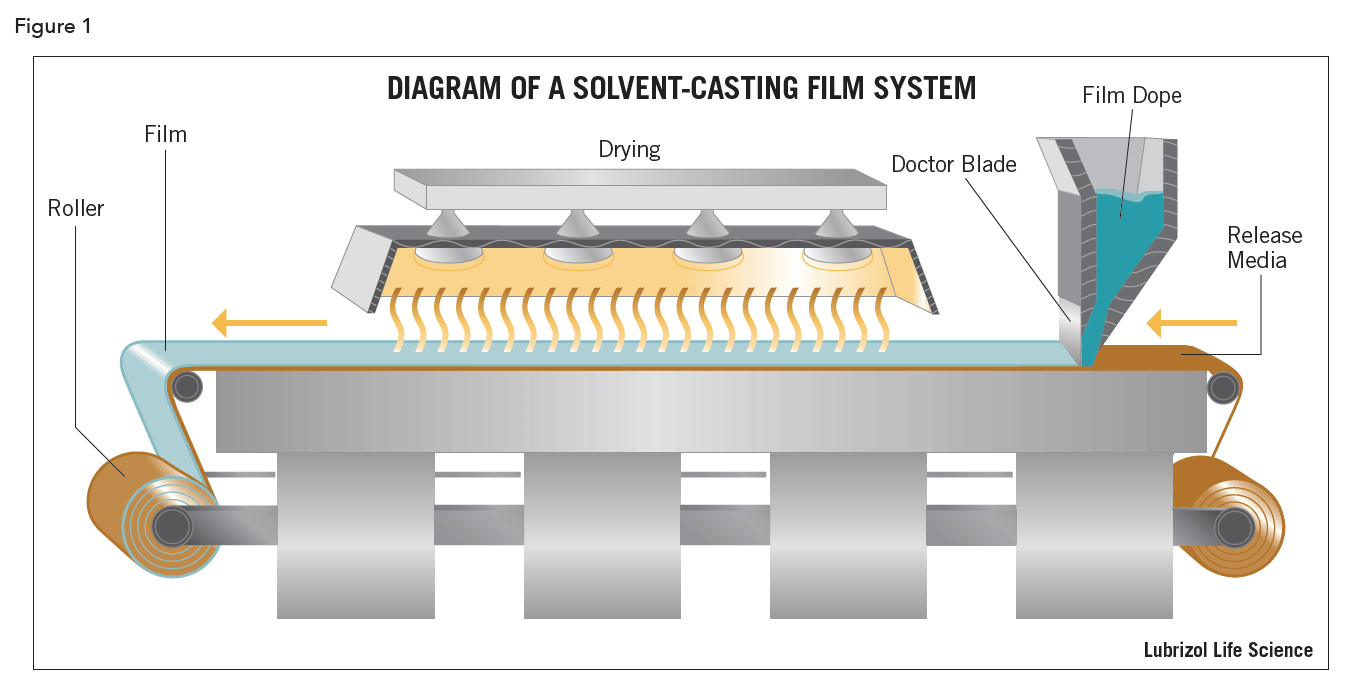
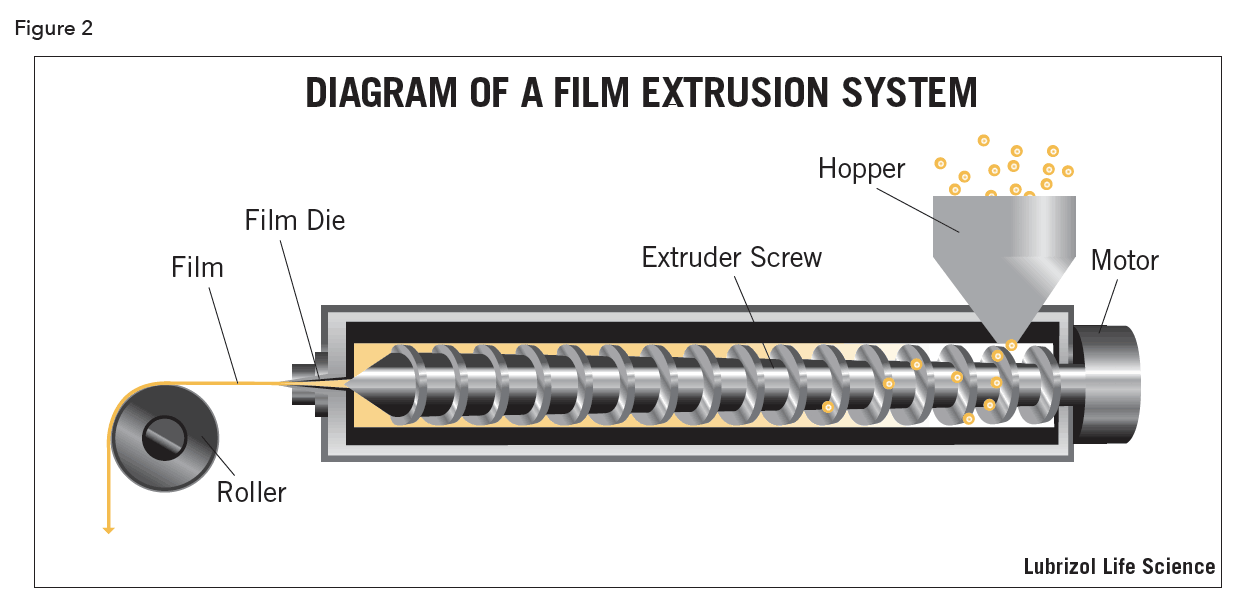
Solvent-Cast Films
Solvent-casting is a century- old film-making process. For pharmaceutical applications, the API is either suspended or dissolved in a solution of polymers, plasticizers, and any other ingredients dissolved in a volatile solvent, like water or ethanol. This material, referred to as the film dope, is spread out using classical solvent-cast film deposition methods onto a continuous roll of release media, such as plastic- impregnated paper. The coated media is passed through a drying apparatus, such as an oven or a convection chamber, to drive off the solvents. The dried film is then die cut into strips and packaged individually in sealed, atmosphere-resistant pouches.
Solvent-casting is ideal for manufacturing films containing heat-sensitive APIs because the temperatures required to remove the solvents are relatively low compared to those needed for a hot-melt extrusion process. However, the dried solvent-cast films can contain trace amounts of residual solvents, which could present issues with compendial compliance. Also, if any of the solvents used are flammable, like ethanol, special safety equipment and procedures must be employed to prevent fire and environmental hazards from vaporized solvent.
Extruded Films
In hot-melt extrusion, the dry ingredients for the film are heated and homogenized by the action of an extruder screw until they are molten and mixed. The melted material is forced through a flat extrusion die that presses the extrudate into the desired film shape. The thickness and strength of the film can further be affected by elongation rollers while the material is still hot and pliable. The extruded film is then cooled, cut and packaged.
The primary drawback of hot-melt extrusion is that it subjects the film ingredients to high temperatures, which could cause thermal degradation. All the ingredients that are used in a hot-melt film must also be devoid of water or any other volatile solvents. The heat of processing will cause such contaminants to boil and create voids in the film, affecting its uniformity, strength and appearance.
Conclusion
Oral dosage forms remain the primary delivery route for pharmaceuticals because of ease of administration and beneficial release characteristics. However, the pharmaceutical industry’s interest has increased in delivery forms that administer medicines directly through vehicles, like transdermal patches, and drug-impregnated medical devices, referred to as drug-eluting devices or implantable drug delivery systems. The rapidly dissolving film drug delivery vehicle bridges the gap between the two ideas, incorporating positive elements from both solid and liquid dosage forms into an elegant, stable, and effective delivery vehicle.