
Encapsulation
Table of Contents
Active pharmaceutical ingredients (APIs) are often administered as aqueous solutions or suspensions, and pharmacokinetics are primarily determined by drug concentration or particle size. Sometimes a formulation requires properties such as taste-masking, physicochemical protection of fragile APIs, and extended API release. API-loaded microparticles and nanoparticles are one method used to achieve these properties. Another use for this technology is when formulating a combination drug product with two different APIs that are not compatible with on another.
Excipients used to prepare such particles include polymers (usually polyesters such as poly(L-lactic-co-glycolic acid) and polycaprolactone, lipids (typically phospholipids, triglycerides, and natural waxes), or insoluble metal salts and oxides, such as silica, calcium phosphate, and calcium carbonate.
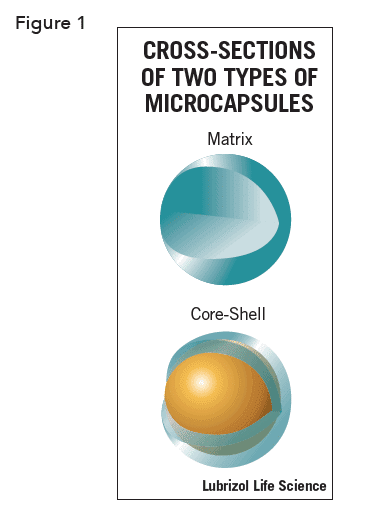
This technical brief will focus on ways to prepare and characterize drug-loaded microcapsules with two types of morphology, matrix style and core-shell. Matrix style microcapsules have an API distributed homogeneously throughout the microcapsule and core-shell microcapsule have an outer coating with the API encapsulated within (see Figure 1). Additionally, Various processes, and the properties of the capsules they yield, are in Table 1.
Matrix Microcapsules Solvent Cast/Grind
A simple way to prepare API- loaded matrix microcapsules is to dissolve the API and the particle- forming excipient in a solvent, remove the solvent to produce a slab of drug-loaded excipient, then grind the slab to produce a powder of drug-loaded particles. The volatile organic solvents must be removed from the final product to acceptably safe levels. In the case of lipidic excipients, casting the slab from a mixture of API and molten excipient avoids the use of solvents. If the API is soluble in the excipient, then homogeneous particles result from grinding the slab, otherwise inhomogeneous distribution can result. This may be minimized by first micronizing the API to a size much smaller than the final desired microcapsule.
Spray Processes
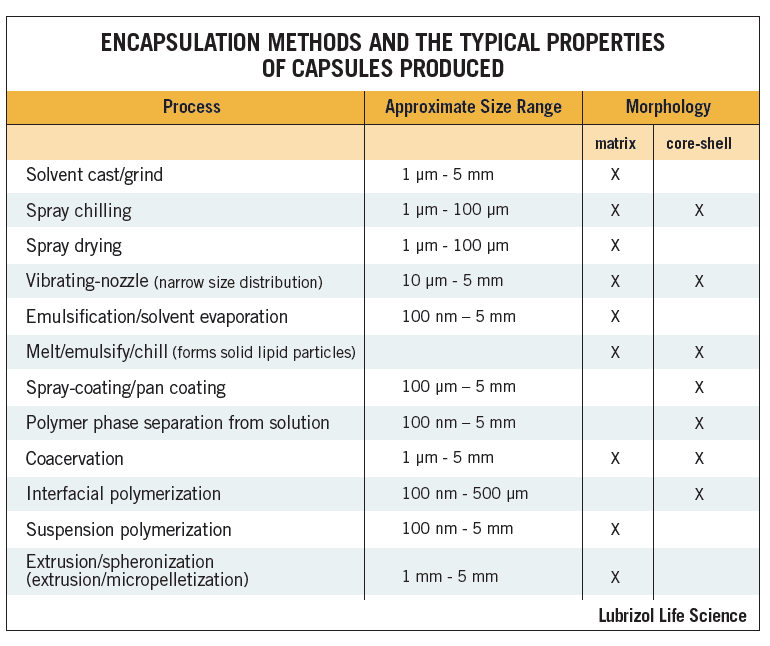
An alternative method to make particles from a solution of API and excipient is spray drying. During this process, the API/excipient/solvent solution is atomized through a heated nozzle into a chamber where the solvent evaporates from the droplets to yield solid particles (see Figure 2)1. The solvent is recovered for disposal or recycling, and the particles are collected in a cyclone. Particles are usually approximately spherical, and in the size range of 1 – 50 μm. For matrix excipients that melt at relatively low temperatures, such as waxes and lipids, solvent-free spray-chilling can be used.
 During this process, the API and excipients are co-melted and sprayed in molten form through the nozzle and the particles harden upon cooling. Spraying under laminar flow conditions from a vibrating nozzle yields particles with a very narrow size distribution.
During this process, the API and excipients are co-melted and sprayed in molten form through the nozzle and the particles harden upon cooling. Spraying under laminar flow conditions from a vibrating nozzle yields particles with a very narrow size distribution.
Emulsion-based Processes
For water-insoluble APIs, the API/excipient/solvent solution can be emulsified into an aqueous surfactant solution using industry standard emulsification equipment, such as overhead paddle mixers, rotor-stator homogenizers, and inline static mixers. Precise control of droplet size can be achieved with emulsification techniques such as tangential-flow membrane (TFM) emulsification, microfluidics, and vibrating nozzles2. Emulsions are made by TFM by forcing the organic phase through the pores of a membrane separating it from a tangentially flowing aqueous emulsifier phase. Flow-rates and pore size, shape, angle, and surface-chemistry control the droplet size and distribution. Vibrating nozzles and microfluidic devices can also form particles with a very narrow size distribution. In all emulsion-based processes, the organic solvent must be relatively insoluble in water, and preferably have a low boiling point for easy removal. Methylene chloride is often the solvent of choice, though ethyl acetate is also used since it can be readily removed by dilution due to its higher water solubility (~8%) and has lower toxicity than chlorinated solvents. If low melting point particle-forming excipients are used, the molten excipient/API phase can be emulsified and the solid-lipid microcapsules form upon cooling without the use of organic solvents.
 In a related process, alginate- based microcapsules can be made without the use of any organic solvents. An aqueous solution of sodium alginate containing API is dripped into a solution of Ca2+ ions. The divalent metal ions cause the dissolved alginate polymer to gel and form particles. This process has even been used to encapsulate live cells and bacteria3.
In a related process, alginate- based microcapsules can be made without the use of any organic solvents. An aqueous solution of sodium alginate containing API is dripped into a solution of Ca2+ ions. The divalent metal ions cause the dissolved alginate polymer to gel and form particles. This process has even been used to encapsulate live cells and bacteria3.
Hot-Melt Extrusion (HME)
API and thermoplastic excipients can be intimately mixed without solvent under high shear and elevated temperature using co-rotating intermeshing screws of a hot-melt extruder. The extruded ribbon can be micropelletized, ground, or spheronized to produce final API-loaded particles. API/microcrystalline cellulose microparticles are made this way for filling into gelatin capsules.
Since matrix particles contain API homogeneously distributed throughout the particle, material at the surface can be released too quickly, be degraded, or impart unpleasant taste, and release is not constant over time. Core-shell particles offer more control.
Core-shell Microcapsules
Core-shell microcapsules are useful when no active material is desired at the particle’s surface. This may be for taste-masking, chemical protection of the active, or control over release kinetics. For oral applications, coating API particles with an excipient that is insoluble in the stomach, but soluble at the elevated pH of the lower intestine, can release the API where desired. For enteric coatings, excipients such as acrylic Eudragit™ polymers and cellulose acetate phthalate are used. The shells can be deposited on solid or liquid cores.
Spray Coating and Pan Coating
The simplest core-shell microcapsule is a particle of an active substance coated with an excipient. The API particles can be coated by spraying a solution or suspension of the excipient into a fluidized bed filled with core particles4. Tablets can be encapsulated in enteric polymers by spraying them with enteric coating solution or dispersion in a V-blender, or by pan-coating where the tablets are agitated in a hot “pan” containing molten coating excipients.
Polymer Phase-separating from Solution
API particles can be coated by precipitating a dissolved polymer onto the surface of co-dispersed API particles, facilitated via either temperature reduction or addition of a polymer non-solvent. Polyisobutylene has be used as a co-phase inducer and final particle stabilizer5. Microcapsules containing liquid cores are generally produced from oil-in-water emulsions.
Processes to encapsulate droplets
Coacervation
Oil-in-water emulsion droplets are coated in polyelectrolytes (such as gelatin and gum-Arabic) by coacervation. At specific pH and concentrations, the polymers form a complex that coats the emulsion droplets that can be chemically hardened to form a shell
Interfacial Polymerization
Monomers dissolved in the oil droplets can react with others dissolved in the aqueous phase to build a wall at the interface. Walls made of polyurethane, polyester and polyamide are most common. Droplets may also be encapsulated by polymerization of urea and formaldehyde dissolved in the aqueous phase.
Phase Separation in Emulsions
Removal of a low-boiling organic solvent from emulsified droplets containing the solvent, shell forming polymer, active ingredient, and high-boiling non-solvent, can yields microcapsules when the low boiling solvent is removed if the emulsifier is chosen properly6.
Concentric Nozzles
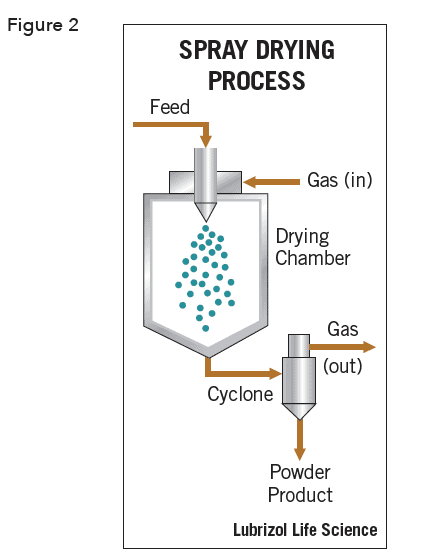
The same vibrating nozzle discussed earlier can be configured to yield monodisperse core-shell particles. The nozzle is concentrically aligned within a second nozzle and core material is ejected from the inner nozzle while shell material is ejected from the outer nozzle (Figure 3). Drying the resulting droplets yields core-shell microcapsules.
 Conclusion
Conclusion
Encapsulation provides pharmaceutical developers with a versatile and commercially validated formulation technique. It can be useful for both drug product intermediates and final products when faced with the oral delivery of foul-tasting APIs, fragile APIs, or combining two incompatible APIs into a combination product. Encapsulation is also becoming ever more popular for long-acting dosage forms for the extended API release.
References
- Formulating Drug Delivery Systems by Spray Drying, Maria-Inês Ré, Drying Technology: An Inter- national Journal, 1532-2300, 24, (4) 433 – 446 (2006)
- The Membrane Emulsification Process—a Review, Charcosset , Limayem I., Fessi H., Journal of Chemical Technology & Biotechnology, 79 (3) 209-218 (2004)
- Encapsulation of Lactic Acid Bacteria in Calcium Alginate Beads for Bacteriocin Production, Evelina Ivanova, Valentina Chipeva, Iskra Ivanova, Xavier Dousset, Denis Poncelet, Journal of Culture Collections, 3 53-58 (2000- 2002)
- Qualitative Description of the Wurster-Based Fluid-Bed Coating Process, Norring Christensen and P. Bertelsen, Drug Development and Industrial Pharmacy, 23 (5) 451-463 (1997)
- Microencapsulation by Ethyl-cellulose Phase Separation: Microcapsule Characteristics, Chemtob, J.C. Chaumeil, M. N’Dongo, International Journal of Pharmaceutics, 29 (1) 1-7 (1986)
- Preparation of Poly (methyl-methacrylate) Microcapsules with Liquid Cores, Loxley , Vincent B., Journal of Colloid and Interface Science, 208 (1) 49-62 (1998)