
Phase Behavior of Surface-Active Solutes
Table of Contents
Surface-active agents (surfactants) are ubiquitous in industrial, cosmetic, and pharmaceutical products. Living systems also use surfactants, such as those that are naturally occurring like lecithin and phospholipids. Most discussions of surfactants are concerned with relatively low concentrations so that the system contains simple surfactant species, such as monomers and their basic aggregates (micelles). However, this is only a part of the total phase behavior of binary systems containing water and a surfactant. Many such solutes form elaborate structures at higher concentrations. The milky appearance of soap solutions does not arise because of micelles (they are too small to scatter a significant quantity of light) but is due to the presence of larger entities termed mesomorphic phases (mesophases). These are anisotropic structures in which one or two dimensions are highly extended.
In the food industry, certain surfactants are used as emulsifiers, including the Spans and Tweens, which are used extensively and can form mesophases in water. In addition, oil lipids (monoglycerides, phospholipids and sterol esters) are used and can also form mesophases that promote emulsion stability and stabilize aerated systems (i.e., whipped cream), improve consistency, texture and shelf-life, and modify rheological properties.
Surfactant mesophases are lyotropic, i.e., their structure is determined by specific interactions between the surfactant molecules. Natural fatty-acid soaps and phospholipids can also form thermotropic mesophases1; here the properties are determined by the temperature of the system. The various lyotropic structures that may occur as the concentration of a solute increases in a binary aqueous system are shown schematically in Figure 11. When the surfactant concentration in aqueous solution exceeds ~10% by weight, micelle-micelle interactions become significant and the simple spherical structures generally undergo conversion first to infinite cylinders and then to multi-bilayers. The middle phase consists of surfactant molecules grouped into rod-like clusters of indefinite length. These are arranged in a hexagonal packing arrangement comprising, typically, an oil-core where the lipophilic groups form the core and hydrophilic groups lie on the surface; it exhibits optical birefringence (opalescence). Most surfactant/water systems are of this type. In the viscous isotropic phase, the molecules pack in spheres that then assemble into a face-centered or body-centered cubic lattice structure. Cubic phases are the most difficult to detect and characterize and are the least well-known. However, they are useful in drug delivery. The neat phase possesses a lamellar structure in which water is sandwiched between the layers of amphiphilic molecules. Molecules with lamellar packing may be perpendicular to the plane or tilted; the angle of tilt is a function of the water content. Except for the fact that the chains have a disordered conformational structure, this phase closely resembles the typical crystalline state of pure surfactant. The level of order can vary. For example, four different neat lamellar phases have been identified in the dipalmitoylphosphatidylcholine water system. The terms “middle” and “neat” phases were originally described by 19th century soap-boilers3.
The phase behavior of concentrated surfactant solutions is complex and is strongly influenced by the molecular geometry of the surfactant4,5. This latter aspect is of considerable importance to biological membranes6. It is often tacitly assumed that surfactant solutions are in instantaneous equilibrium with respect to micelle, or other structure, formation when changes in conditions (degree of dilution, temperature, salt addition, etc.) are made. However, the time(s) required for the formation of, or disruption to, an equilibrium phase structure in concentrated surfactant solutions may require several days7.
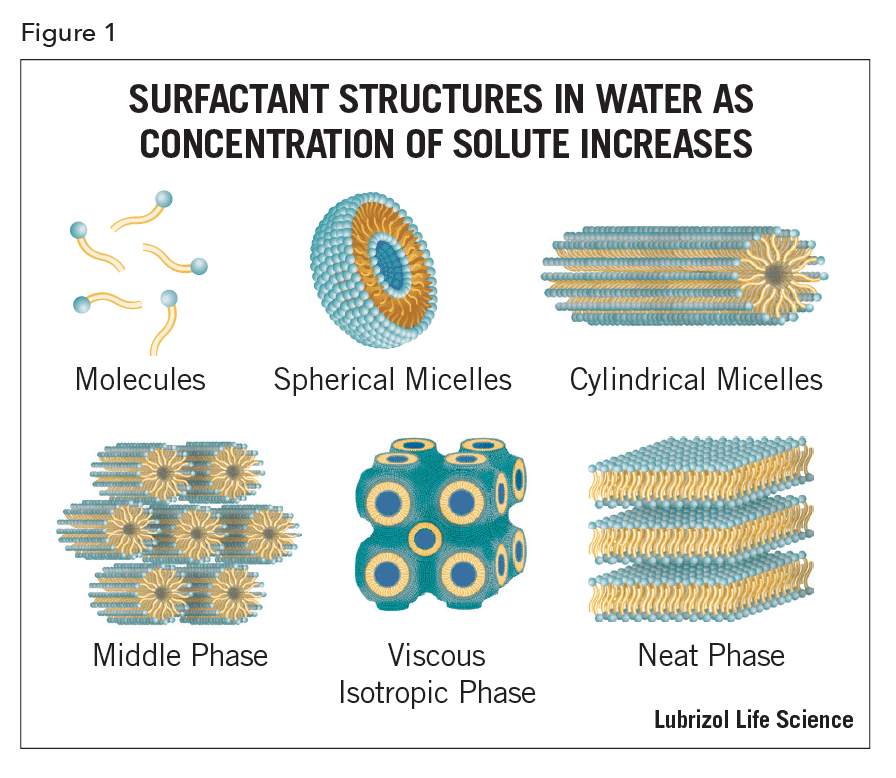 A mesophase is an in-between, or intermediate, phase that exhibits certain aspects of both solid and liquid states while also possessing properties that are not found in either solids or liquids. For example, it has characteristic of crystals (such as birefringence) and yet can flow like a liquid, hence the term liquid crystal (LC)8. Their application in LC displays depends on the ability to change orientation as a function of applied electric field; a function that requires both structure and fluidity. The structures of LCs are determined by putting them in small (~1 mm) capillaries and examining them using a microscope with crossed polarizers. One of the important recent applications of the various fluid micro and nano-structures that can be created from the spontaneous assembly of surfactants in solution is the creation of “nanocomposites”9.
A mesophase is an in-between, or intermediate, phase that exhibits certain aspects of both solid and liquid states while also possessing properties that are not found in either solids or liquids. For example, it has characteristic of crystals (such as birefringence) and yet can flow like a liquid, hence the term liquid crystal (LC)8. Their application in LC displays depends on the ability to change orientation as a function of applied electric field; a function that requires both structure and fluidity. The structures of LCs are determined by putting them in small (~1 mm) capillaries and examining them using a microscope with crossed polarizers. One of the important recent applications of the various fluid micro and nano-structures that can be created from the spontaneous assembly of surfactants in solution is the creation of “nanocomposites”9.
Phase Diagrams
A phase diagram is a graphical portrayal of information that answers three basic questions:
(i) how many phases exist? (ii) what is the qualitative nature of each phase? and (iii) what is the composition of each phase? From a properly constructed diagram one can obtain, by inspection or calculation, the answers. Other kinds of information can also be deduced, such as the proportion of the total system in each of the coexisting phases and the temperature dependence.
Phase diagrams are used to specify the temperature and concentrations at which various structures exist at equilibrium. In binary diagrams, by convention, temperature is placed on the ordinate and composition on the abscissa, with water to the left and surfactant to the right. Figure 2 is a binary diagram of condensed phases of an alkyl polyoxylated (C12 E6) nonionic solute in water; the lines designate the experimental boundaries: 2L is two isotropic liquids; S is one isotropic liquid; M is a middle phase; I+M is ice plus middle phase; I+C is ice plus crystals; N is a neat phase.
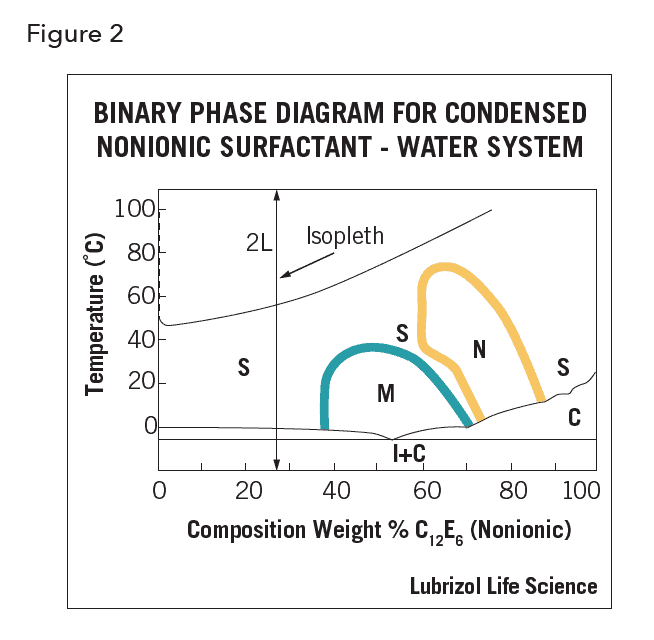 The middle and neat LC phases occur at high concentrations. The more extensive they are, on a phase diagram, the longer the alkyl chain. A vertical line of constant composition but variable temperature is termed an isopleth. The boundaries that delineate the range of existence of the various phases assume distinctive shapes; typically, the region is bullet- or dome-shaped. The same LC states found within surfactant water systems are also found within surfactant – electrolyte water systems.
The middle and neat LC phases occur at high concentrations. The more extensive they are, on a phase diagram, the longer the alkyl chain. A vertical line of constant composition but variable temperature is termed an isopleth. The boundaries that delineate the range of existence of the various phases assume distinctive shapes; typically, the region is bullet- or dome-shaped. The same LC states found within surfactant water systems are also found within surfactant – electrolyte water systems.
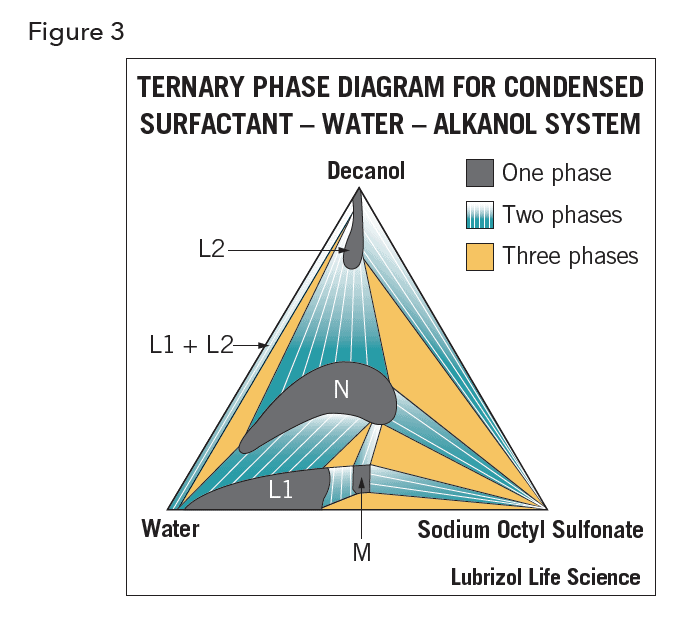
In ternary systems in which the third component is an alkane or fatty derivative (such as an acid, alcohol, or amide) the same associated structures can be identified. Indeed, adding an “oil” to a surfactant-water system often forms a more complete set of LC states than is possible without the oil. Figure 3 illustrates the ternary system of water sodium octyl sulfonate decanol (at 20 oC). The surfactant has limited solubility both in water and decanol but the alcohol is not soluble in water. The aqueous phase, L1, contains hydrophilic micelles; at concentrations below the critical micelle concentration (CMC) it contains no decanol. The alcohol phase, L2, contains inverse (lipophilic) micelles. The phases N (neat, displaying lamellar structure) and M (middle, displaying two-dimensional hexagonal structure) are both liquid crystals. This diagram contains features that occur as general behavior in all such ternary systems of surface-active solutes, water, and fatty-acid derivatives. Changes of temperature, salinity, fatty-acid derivative, and structure of the surfactant cause systematic shifts in the relative positions of the one-phase regions. The phase behavior of water-oil-surfactant systems represented using a ternary diagram can be used to determine the composition required to create, for example, a microemulsion10. Once the composition is determined, microemulsions are easy to manufacture reproducibly; they are also transparent and behave like a clear liquid with low viscosity and, unlike coarse emulsions, they are thermodynamically, i.e. indefinitely, stable11.
 The experimental methods for determining phase information, analyzing two- and three-component phase diagrams, and calculating phase ratios is beyond the scope of this brief; an extensive and in-depth description of these matters can be found in reference8.
The experimental methods for determining phase information, analyzing two- and three-component phase diagrams, and calculating phase ratios is beyond the scope of this brief; an extensive and in-depth description of these matters can be found in reference8.
Phase information is possibly the most useful kind of physical data that exists for surfactant systems. A few practical examples serve to illustrate this:
- The trajectory of any physical process in which composition and/or temperature are varied (within a defined system) may be charted on that system’s phase Heating or cooling any system of fixed composition corresponds to rising or falling along an isopleth in a phase diagram (Figure 2). The evaporation of solvent from a solution corresponds to moving to the right; dilution of a system is depicted by moving to the left.
- Phase heterogeneity underlies most separation processes. In soap-making, the hot “neat” liquid crystal phase is used to form framed soap bars after separation from the lye phase and cooling. In fat refining, polar lipid impurities are removed from fats by first precipitating the liquid crystal and then removing it by
- Phase behavior dictates the selection of suitable components for use in a product. Components of liquid detergents, which are ordinarily concentrated solutions, must be selected with both phase behavior and utility in mind.
- The utility of surfactants is often limited by inadequate solubility; phase information provides a complete (both qualitative and quantitative) Whenever a solubility boundary lies well below an intended use concentration it will result in impaired effectiveness of the material for the intended use.
- Control of “interfacial properties” via control of bulk phase composition is possible. Foam stabilization is exceptionally good when the system has two phases, one being a small amount of a lamellar LC
- Perturbations in hydrophilic or lipophilic group structural features alter phase boundary locations in surprisingly predictable ways. Phase behavior can distinguish non-surfactant amphiphilic molecules from true surface-active
Conclusion
In conclusion, the existence of predictable relationships between phase behavior and structure is potentially very significant. When the need for a specific kind of phase behavior can be stated, molecules can then be efficiently selected or designed to fill this need.
References
- Dickinson and G. Stainsby, Colloids in Food, Applied Science Publishers, London (1982)
- Bourrel and RS. Schechter in Microemulsions and Related Systems, Surf. Sci. Series 30, Marcel Dekker, New York (1988)
- Corkhill and J Goodman, Adv. Coll. Int. Sci., 2 2297 (1969)
- Laughlin, The Aqueous Phase Behavior of Surfactants, Academic Press, London (1994)
- Israelachvili, Intermolecular and Surface Forces, Academic Press, London (1985)
- Friberg (ed), Liquid Crystals and the Structure of BioMembranes, Adv. Chem. Series, ACS Publications, Washington DC (1976)
- Zana, Dynamics of Surfactant Self-Assemblies: Micelles, Microemulsions, Vesicles and Lyotropic Phases, Surf. Sci. Series 125, Marcel Dekker, New York (2005)
- Priestly (ed), Introduction to Liquid Crystals, Plenum Press, New York (1974)
- Dagami, Chem&Eng News, 77 (23) 25 (1999)
- Fanum (ed), Microemulsions: Properties and Applications, CRC Press, Boca Raton FL (2008)
- Nakajima, in Industrial Applications of Microemulsions, Surfactant Science Series 66, (ed) C. Solans, Marcel Dekker, New York (1997)