
Solid Lipid Nanoparticles
Table of Contents
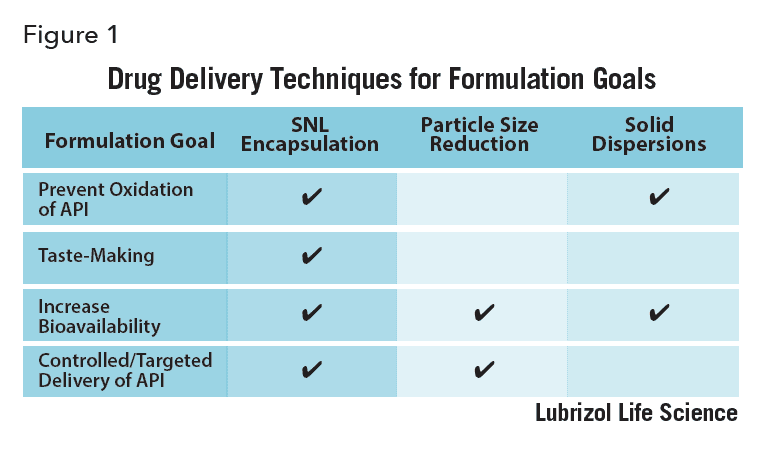
Formulators of active pharmaceutical ingredients (APIs) are often trying to find ways to prevent aerial oxidation, mask an unpleasant taste, increase bioavailability, or control the way an API is delivered (to target a particular cell, tissue, or organ, for example).
There are various formulation techniques that can help overcome these four challenges. Taste masking or protecting an API from degradation often requires encapsulating the API in a tasteless protective material. Improving the solubility and bioavailability of BCS class II and IV APIs can be achieved via particle size reduction[1], amorphous solid dispersions, and encapsulation, among other technologies. API targeting of cells and tissues has been demonstrated by incorporating the API within nanoparticles designed to recognize targets by engineering specific molecular interactions between particles and the target. While each of these techniques is valuable, they only solve one or two formulation challenges.
Solid lipid nanoparticles (SLNs) are aqueous dispersions of submicron particles made of waxy or lipid materials that are solid at body temperature[2] and are useful to deal with all four challenges. They can be formulated with APIs as diverse as poorly water soluble small molecules to high molecular weight water soluble ones like proteins, peptides and nucleic acids. SLNs have been used for decades to reduce oxidative degradation of vitamins encapsulated within them[3] and to improve the UV-absorbing properties of organic sunscreen molecules[4]. Since the 1990s, SLNs have been studied as drug delivery vehicles alongside other nanocarriers such as liposomes and polymer nanoparticles. Recently, SLNs have been investigated as carriers of antigenic proteins in novel vaccine research[5]. As such, SLNs offer a versatile approach to solve several drug delivery issues and deserve a closer look.
In this brief, we explore how these useful and versatile nanoparticles are made and characterized, how various types of API are incorporated into them, and discuss examples of their use in drug delivery.
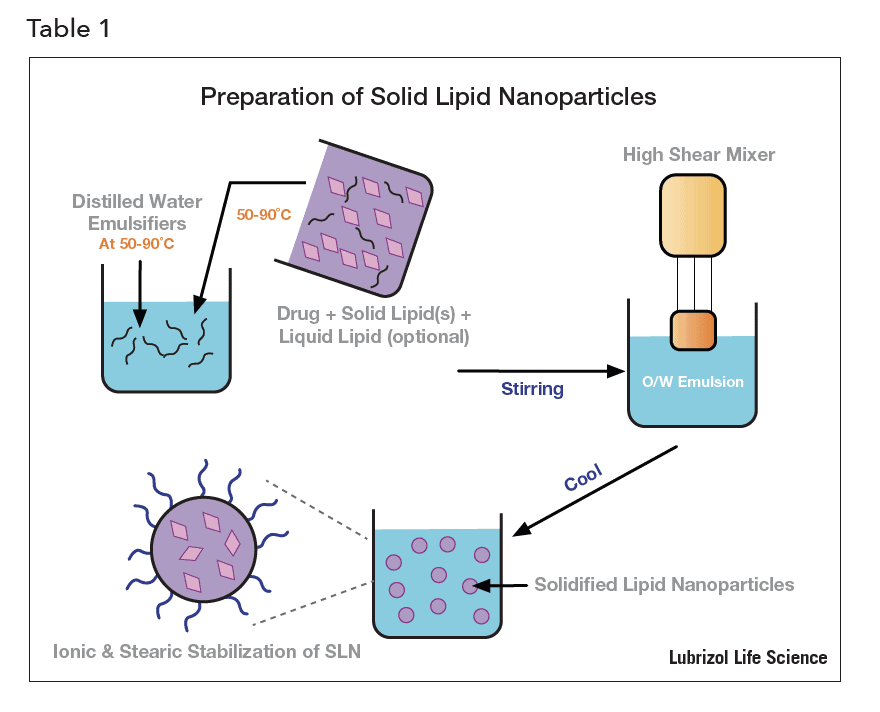 Manufacturing
Manufacturing
SLNs are prepared by mixing a lipid excipient with an aqueous emulsifier solution above the melting temperature of the lipid excipient, to produce a hot oil-in-water emulsion that is then cooled to form the solid nanoparticles. Commonly explored lipid excipients are: fatty acids, fatty alcohols, partial glycerides, triglycerides and waxes (e.g. carnauba wax or bees wax).
 An emulsifier is used to reduce the interfacial tension between the molten lipid and the water, facilitating the breakup of the liquid lipid into small droplets under the high shear mixing forces. With the correct choice of emulsifier, a high shear device such as colloid mill, ultra-sonicator or high-pressure homogenizer, can reduce the average size of the dispersed molten lipid droplets to the nanometer range. Once the desired particle size distribution is achieved, the nanoemulsion is cooled to room temperature to solidify the droplets into SLNs. Emulsifier molecules tend to adhere (adsorb) at the SLN particle surface and become physically trapped there as the wax solidifies. Therefore, the nature of the emulsifier molecule dictates the surface properties of the SLNs and how they behave both in vitro and in vivo. Selecting the right emulsifier is critical, not only for emulsion formulation, but also to control the properties and function of the SLNs.
An emulsifier is used to reduce the interfacial tension between the molten lipid and the water, facilitating the breakup of the liquid lipid into small droplets under the high shear mixing forces. With the correct choice of emulsifier, a high shear device such as colloid mill, ultra-sonicator or high-pressure homogenizer, can reduce the average size of the dispersed molten lipid droplets to the nanometer range. Once the desired particle size distribution is achieved, the nanoemulsion is cooled to room temperature to solidify the droplets into SLNs. Emulsifier molecules tend to adhere (adsorb) at the SLN particle surface and become physically trapped there as the wax solidifies. Therefore, the nature of the emulsifier molecule dictates the surface properties of the SLNs and how they behave both in vitro and in vivo. Selecting the right emulsifier is critical, not only for emulsion formulation, but also to control the properties and function of the SLNs.
Anionic emulsifiers such as sodium dodecyl sulfate result in SLNs with negatively charged surfaces, which can attract positively charged molecules that get bound at the particle surface. Cationic emulsifiers such as cetyl trimethylammonium bromide lead to SLNs with positively charged surfaces that can bind negatively charged molecules at the surface. Non-ionic emulsifiers such as Tween 20 and Tween 80, result in SLNs with virtually no surface charge, and tend not to bind any molecules at all. Additionally, PEGylated excipients may be included in the mix and may lead to formulations that can avoid the reticuloendothelial system, while providing a longer in vivo half-life.
A note of caution when developing uncharged SLNs – many non-ionic emulsifiers that work well at room temperature become increasingly insoluble in water at higher temperatures and have the tendency to then precipitate from the hot aqueous phase at a temperature called the emulsifier’s “cloud point” (the temperature above which an aqueous solution of a water soluble surfactant becomes turbid). Non-ionic surfactants must be chosen carefully, with cloud point compared to the maximum temperature that the nanoemulsion will experience during manufacturing.
The presence of emulsifiers at the SLN surface also overcomes the long range attractive Van der Waals forces that they experience in a dispersion, which would otherwise lead to particle aggregation. The surface charge imparted to SLNs made with ionic emulsifiers can be sufficient to produce an electrostatic repulsive force, whereas non-ionic emulsifiers trapped at the particle surface can produce a physical or “steric” repulsive force between SLNs. In each case, SLNs can be made whose dispersion stability is suitable for the shelf life of a pharmaceutical product.
SLN formulations for parenteral administration must be sterile. If the SLNs are less than 100 nm in diameter, it may be possible to sterilize them after manufacturing by filtration via a 0.2-micron filter. However, this is usually not the case. In other cases, the formulations may be amenable to terminal heat sterilization, but is also rare. Although much more challenging than terminal sterilization, aseptic manufacturing, using sterile excipients and equipment, as well as stringent controls to protect from microbial ingress during processing, is usually the only option.
API Incorporation into SLNs
Small lipophilic molecules are ideally suited to the SLN approach if the API is soluble in the molten lipid. In this case, the API is mixed with the molten lipid prior to nanoemulsification. A further requirement for a stable product is that the API remain dissolved in the SLNs once the nanoemulsion precursor is cooled. In some cases, the API is soluble in the molten lipid, but upon cooling, it precipitates – either because the API is not soluble in the solid lipid, or because the lipid crystalizes within the solidifying SLNs, forcing the API out of the nanoparticles. In such cases, macroscopic crystals of the API can be observed by optical microscopy in the aqueous phase around SLNs. One remedy for this precipitation is the incorporation of a liquid lipid along with the solid lipid used to prepare the nanoemulsion. The second lipid is chosen so that it remains liquid after the SLNs solidify on cooling, and can either prevent the solid lipid from crystallizing, thus preventing API expulsion, or can form liquid domains within the SLN nanoparticles into which the API can remain dissolved. SLNs containing a liquid lipid to increase API solubility are called nanostructured lipid carriers (NLCs)[6], and API loadings of up to 10 or 20 wt% are possible this way.
 Large molecules such as proteins and nucleic acids are best incorporated at the SLN surfaces. Unlike other nanoparticle delivery vehicles, covalent bonds between the nanoparticle and the API are not required – the electrostatic surface charge resulting from the use of ionic emulsifiers is generally sufficient to achieve high surface loadings, and the all-important conformation of protein APIs can be well preserved.
Large molecules such as proteins and nucleic acids are best incorporated at the SLN surfaces. Unlike other nanoparticle delivery vehicles, covalent bonds between the nanoparticle and the API are not required – the electrostatic surface charge resulting from the use of ionic emulsifiers is generally sufficient to achieve high surface loadings, and the all-important conformation of protein APIs can be well preserved.
Nucleic acids are negatively charged and can only be attached to the surface of positively charged SLNs, however proteins with a net charge at physiological pH can surprisingly bind to SLNs of the same sign of charge, as well as the expected oppositely charged ones. This has been ascribed to the fact that proteins with a net negative or positive charge have some domains that are oppositely charged, and these domains can interact with the charged SLN surface. Furthermore, interactions between hydrophobic domains of the protein and the hydrophobic SLN surface (or hydrophobic residues on the SLN surface bound emulsifier molecules), have been shown to provide an additional mode for protein adhesion. In contrast, SLNs made with non-ionic emulsifiers tend not to bind any proteins at all, regardless of the protein charge nature, as a result of the same repulsive force that keeps such SLNs from adhering to one another, as discussed previously.
Characterization
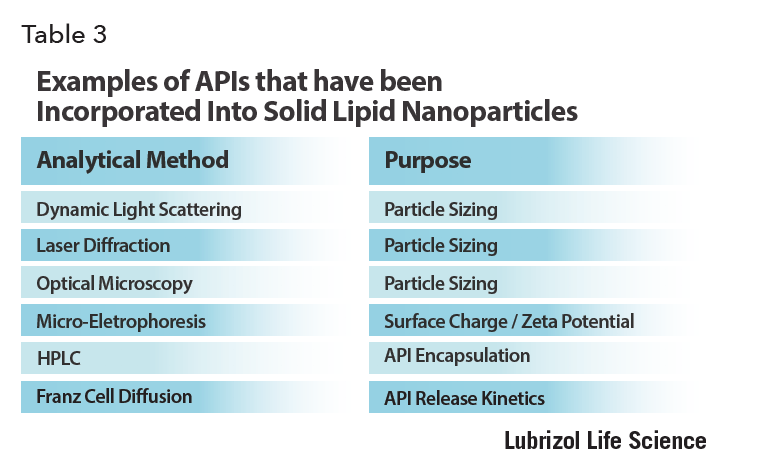
The particle size distribution of SLNs can be measured by techniques borrowed from the colloid science field for characterizing model nanosuspensions – that is, dynamic light scattering (DLS) and laser diffraction (LD). DLS results are most accurate when the sample has a narrow size distribution and particles smaller than about one micron in diameter. Laser diffraction on the other hand, is suitable for samples with broader size distributions, although if particles bigger than about one micron are present, the sample must be continually agitated during the measurement to prevent their sedimentation. Both techniques rely on dilution of the sample, and it must be verified that this does not affect the stability of the dispersion before measurements are made.
Of course, optical microscopy – the backbone of all nano- and micro particle system characterization – should always be used to ensure there are no obvious oversized particles in the sample, and certainly no free API crystals. Polarized light microscopy is particularly helpful in this latter regard as API crystals are easily identified under these conditions. Raman microscopy can aid in chemical identification of any large particles observed.
Since SLN surface charge can determine API binding and can have an impact on the in vivo response to the particle, it must be carefully characterized. This is best done by microelectrophoresis. By applying an electric field between two electrodes immersed in the SLN suspension and measuring how the SLNs move in response to it using light scattering, the mean SLN electrical surface charge can be determined from the mean SLN drift velocity.
To determine the amount of API encapsulated by the SLNs for the case of small molecules, high performance liquid chromatography (HPLC) is the best method. The API must be extracted from the SLN into an organic solvent first. For the case of surface bound large molecule APIs, the incorporation efficiency is determined by first removing the SLNs from the dispersion by centrifugal filtration, then quantitating the free API in the aqueous filtrate. Back calculation is then used to deduce the amount of API bound to the SLNs. For this to be accurate, it must be determined that free API is able to collect efficiently in the filtrate, and not adhere to any surfaces such as centrifuge filters. Spike/recovery experiments are of value to assess mass balance.
The in vitro release of API from SLNs can give an estimate of the in vivo performance and can be determined using Franz cells – devices made from two compartments filled with buffer and separated by a semipermeable membrane. SLNs are introduced into the “donor chamber” on one side of the membrane, and HPLC analysis of the buffer in the receptor chamber on the other side over time can be used to determine the kinetics of API release from the SLNs, and flux of the API through the membrane.
SLNs in Drug Delivery
SLNs are suitable for topical (including ocular and mucosal), oral and parenteral routes of administration. For topical formulations, the SLNs may need only to be combined with a suitable topical vehicle to create a final product. Sunscreens and cosmetics have been formulated this way. Hydrogels containing SLNs have been explored for oral transmucosal delivery[7].
 For oral dosage forms the SLN suspension can be consumed by the patient directly, or the suspension may be spray dried or lyophilized before being combined with other excipients for tablet manufacture.
For oral dosage forms the SLN suspension can be consumed by the patient directly, or the suspension may be spray dried or lyophilized before being combined with other excipients for tablet manufacture.
Parenteral dosage forms can use sterile SLN suspensions directly. As previously mentioned, nanoparticles made using non-ionic emulsifiers do not adsorb any proteins. This includes the opsonin proteins in blood that bind to foreign materials and mark them for elimination. Therefore, this type of SLN can circulate for an extended duration after injection. The increased circulation time allows the API to be fully released into the blood before the SLNs are finally eliminated. It also gives chemotherapeutic-loaded non-ionic SLNs an opportunity to accumulate in hypervascularized tumors; the blood vessels feeding such tumors are unusually porous, so if the SLNs are small enough, they can cross through the blood vessels into the tumor. Since there are no clearance pathways from the tumor, the SLNs reside there for an extended period, and release the chemotherapeutic exactly where it is required. This is called the enhanced permeation and retention (EPR) effect[8].
SLNs can be internalized by a wide variety of cells, and when formulated with antigenic proteins can be used to amplify the immune response to the proteins. Macrophages and dendritic cells, which are designed to identify and actively internalize foreign material, digest the protein-loaded particles and present degradation fragments to T-cells in lymph nodes, stimulating enhanced production of antibodies to the proteins so SLNs can be useful in the development of effective vaccines. These can be delivered parenterally or, since there are high concentrations of macrophages and dendritic cells in mucosal tissue, they can even be applied to vaginal or nasal tissues to generate a strong immune response[9].
Regulatory Aspects
As in all pharmaceutical formulations, the excipients used in the manufacture of SLNs must be generally recognized as safe (GRAS) and ideally comply with pharmacopeial monographs. At the very least, they should be made to strict specifications, under processes demonstrated to be well understood and well controlled.
For parenteral SLN formulations, the “globule” fraction (the percentage of particles with diameter larger than five microns) should be determined and minimized. For example, two globule size specifications that the United States Pharmacopeia requires of intravenous lipid emulsions (such as those used for IV nutrition) are a mean globule size of < 500 nm, and a percentage of lipid globules larger than five microns must be less than 0.05%. It can be expected that regulators would also require similar specifications for parenteral SLNs. Finally, since SLN dispersions are aqueous and can support biological growth, they must be sterilized after manufacture, or contain preservatives to prevent the growth.
Conclusion
SLNs provide a versatile tool for encapsulating and delivering both small molecule hydrophobic APIs and large hydrophilic ones. SLNs have already found commercial application in topical products, and due to their low cost, ease of manufacture, and versatility, are sure to become more commonly found in future pharmaceutical products.
References
- [1] Particle Size Reduction in Water-Insoluble Drug Formulation. in Chapter 17 Particle Size Reduction (ed. Liu, R.) (CRC Press, Taylor & Francis Group, 2018).
- [2] Shah, R., Eldridge, D., Palombo, E. & Harding, I. Lipid Nanoparticles: Production, Characterization and Stability. (Springer International Publishing, 2015).
- [3] Loxley, A. & Fairhurst, D. Micro-and Nano-encapsulation of Water-and Oil-soluble Actives for Cosmetic and Pharmaceutical Applications. (Particle Sciences, Inc.).
- [4] Wissing, S. A. & Müller, R. H. A novel sunscreen system based on tocopherol acetate incorporated into solid lipid nanoparticles. Int J Cosmet Sci 23, 233–243 (2001).
- [5] Stylianou, E. et al. Mucosal delivery of antigen-coated nanoparticles to lungs confers protective immunity against tuberculosis infection in mice. European Journal of Immunology 44, 440–449 (2014).
- [6] Radtke, M., Souto, E. B. & Müller, R. H. Nanostructured Lipid Carriers: A Novel Generation of Solid Lipid Drug Carriers. Pharmaceutical Technology Europe 17, 45–50 (2005).
- [7] Silva, A. C., Amaral, M. H., González-Mira, E., Santos, D. & Ferreira, D. Solid lipid nanoparticles (SLN)-based hydrogels as potential carriers for oral transmucosal delivery of risperidone: preparation and characterization studies. Colloids Surf B Biointerfaces 93, 241–248 (2012).
- [8] Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
- [9] Arias, M. A. et al. Carnauba wax nanoparticles enhance strong systemic and mucosal cellular and humoral immune responses to HIV-gp140 antigen. Vaccine 29, 1258–1269 (2011).